Neural crest origin of olfactory ensheathing glia
- PMID: 21078992
- PMCID: PMC3000254
- DOI: 10.1073/pnas.1012248107
Neural crest origin of olfactory ensheathing glia
Abstract
Olfactory ensheathing cells (OECs) are a unique class of glial cells with exceptional translational potential because of their ability to support axon regeneration in the central nervous system. Although OECs are similar in many ways to immature and nonmyelinating Schwann cells, and can myelinate large-diameter axons indistinguishably from myelination by Schwann cells, current dogma holds that OECs arise from the olfactory epithelium. Here, using fate-mapping techniques in chicken embryos and genetic lineage tracing in mice, we show that OECs in fact originate from the neural crest and hence share a common developmental heritage with Schwann cells. This explains the similarities between OECs and Schwann cells and overturns the existing dogma on the developmental origin of OECs. Because neural crest stem cells persist in adult tissue, including skin and hair follicles, our results also raise the possibility that patient-derived neural crest stem cells could in the future provide an abundant and accessible source of autologous OECs for cell transplantation therapy for the injured central nervous system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
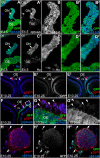

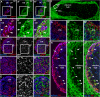
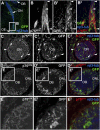

References
-
- Barnett SC, Riddell JS. Olfactory ensheathing cell transplantation as a strategy for spinal cord repair: What can it achieve? Nat Clin Pract Neurol. 2007;3:152–161. - PubMed
-
- Raisman G, Li Y. Repair of neural pathways by olfactory ensheathing cells. Nat Rev Neurosci. 2007;8:312–319. - PubMed
-
- Richter MW, Roskams AJ. Olfactory ensheathing cell transplantation following spinal cord injury: Hype or hope? Exp Neurol. 2008;209:353–367. - PubMed
-
- Kawaja MD, Boyd JG, Smithson LJ, Jahed A, Doucette R. Technical strategies to isolate olfactory ensheathing cells for intraspinal implantation. J Neurotrauma. 2009;26:155–177. - PubMed
-
- Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol. 1985;110:422–439. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

