Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research
- PMID: 21079040
- PMCID: PMC3014230
- DOI: 10.1124/pr.110.003046
Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research
Abstract
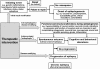
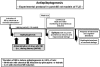
Diverse brain insults, including traumatic brain injury, stroke, infections, tumors, neurodegenerative diseases, and prolonged acute symptomatic seizures, such as complex febrile seizures or status epilepticus (SE), can induce "epileptogenesis," a process by which normal brain tissue is transformed into tissue capable of generating spontaneous recurrent seizures. Furthermore, epileptogenesis operates in cryptogenic causes of epilepsy. In view of the accumulating information about cellular and molecular mechanisms of epileptogenesis, it should be possible to intervene in this process before the onset of seizures and thereby either prevent the development of epilepsy in patients at risk or increase the potential for better long-term outcome, which constitutes a major clinical need. For identifying pharmacological interventions that prevent, interrupt or reverse the epileptogenic process in people at risk, two groups of animal models, kindling and SE-induced recurrent seizures, have been recommended as potentially useful tools. Furthermore, genetic rodent models of epileptogenesis are increasingly used in assessing antiepileptogenic treatments. Two approaches have been used in these different model categories: screening of clinically established antiepileptic drugs (AEDs) for antiepileptogenic or disease-modifying potential, and targeting the key causal mechanisms that underlie epileptogenesis. The first approach indicated that among various AEDs, topiramate, levetiracetam, carisbamate, and valproate may be the most promising. On the basis of these experimental findings, two ongoing clinical trials will address the antiepileptogenic potential of topiramate and levetiracetam in patients with traumatic brain injury, hopefully translating laboratory discoveries into successful therapies. The second approach has highlighted neurodegeneration, inflammation and up-regulation of immune responses, and neuronal hyperexcitability as potential targets for antiepileptogenesis or disease modification. This article reviews these areas of progress and discusses the challenges associated with discovery of antiepileptogenic therapies.
Figures

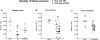

References
-
- Amano K, Hamada K, Yagi K, Seino M. (1998) Antiepileptic effects of topiramate on amygdaloid kindling in rats. Epilepsy Res 31:123–128 - PubMed
-
- André V, Dubé C, François J, Leroy C, Rigoulot MA, Roch C, Namer IJ, Nehlig A. (2007) Pathogenesis and pharmacology of epilepsy in the lithium-pilocarpine model. Epilepsia 48 (Suppl 5):41–47 - PubMed
-
- André V, Ferrandon A, Marescaux C, Nehlig A. (2001) Vigabatrin protects against hippocampal damage but is not antiepileptogenic in the lithium-pilocarpine model of temporal lobe epilepsy. Epilepsy Res 47:99–117 - PubMed
-
- André V, Rigoulot MA, Koning E, Ferrandon A, Nehlig A. (2003) Long-term pregabalin treatment protects basal cortices and delays the occurrence of spontaneous seizures in the lithium-pilocarpine model in the rat. Epilepsia 44:893–903 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

