Temporal changes in the expression of mRNA of NADPH oxidase subunits in renal epithelial cells exposed to oxalate or calcium oxalate crystals
- PMID: 21079197
- PMCID: PMC3145401
- DOI: 10.1093/ndt/gfq692
Temporal changes in the expression of mRNA of NADPH oxidase subunits in renal epithelial cells exposed to oxalate or calcium oxalate crystals
Abstract
Background: Exposure of renal epithelial cells to oxalate (Ox) or calcium oxalate (CaOx) crystals leads to the production of reactive oxygen species and cell injury. We have hypothesized that Ox and CaOx crystals activate NADPH oxidase through upregulation of its various subunits.
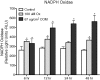
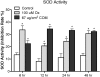
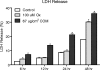
Methods: Human renal epithelial-derived cell line, HK-2, was exposed to 100 μmol Ox or 66.7 μg/cm(2) CaOx monohydrate crystals for 6, 12, 24 or 48 h. After exposure, the cells and media were processed to determine activation of NADPH oxidase, production of superoxide and 8-isoprostane (8IP), and release of lactate dehydrogenase (LDH). RT-PCR was performed to determine mRNA expression of NADPH subunits p22(phox), p40(phox), p47(phox), p67(phox) and gp91(phox) as well as Rac-GTPase.
Results: Exposure to Ox and CaOx crystals resulted in increase in LDH release, production of 8-IP, NADPH oxidase activity and production of superoxide. Exposure to CaOx crystals resulted in significantly higher NADPH oxidase activity, production of superoxide and LDH release than Ox exposure. Exposure to Ox and CaOx crystals altered the expression of various subunits of NADPH oxidase. More consistent were increases in the expression of membrane-bound p22(phox) and cytosolic p47(phox). Significant and strong correlations were seen between NADPH oxidase activity, the expression of p22(phox) and p47(phox), production of superoxide and release of LDH when cells were exposed to CaOx crystals. The expressions of neither p22(phox) nor p47(phox) were significantly correlated with increased NADPH oxidase activity after the Ox exposure.
Conclusions: As hypothesized, exposure to Ox or CaOx crystals leads to significant increases in the expression of p22(phox) and p47(phox), leading to activation of NADPH oxidase. Increased NADPH oxidase activity is associated with increased superoxide production and lipid peroxidation. Different pathways appear to be involved in the stimulation of renal epithelial cells by exposure to Ox and CaOx crystals.
Figures


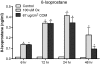
Similar articles
-
Exposure of Madin-Darby canine kidney (MDCK) cells to oxalate and calcium oxalate crystals activates nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase.Urology. 2014 Feb;83(2):510.e1-7. doi: 10.1016/j.urology.2013.10.038. Epub 2013 Dec 19. Urology. 2014. PMID: 24360063 Free PMC article.
-
The effect of calcium on calcium oxalate monohydrate crystal-induced renal epithelial injury.Urol Res. 2009 Feb;37(1):1-6. doi: 10.1007/s00240-008-0160-6. Epub 2008 Nov 13. Urol Res. 2009. PMID: 19005647 Free PMC article.
-
Diphenyleneiodium (DPI) reduces oxalate ion- and calcium oxalate monohydrate and brushite crystal-induced upregulation of MCP-1 in NRK 52E cells.Nephrol Dial Transplant. 2005 May;20(5):870-8. doi: 10.1093/ndt/gfh750. Epub 2005 Mar 8. Nephrol Dial Transplant. 2005. PMID: 15755756
-
Role of the small GTPase Rac in p22phox-dependent NADPH oxidases.Biochimie. 2007 Sep;89(9):1133-44. doi: 10.1016/j.biochi.2007.05.003. Epub 2007 May 17. Biochimie. 2007. PMID: 17583407 Review.
-
NOX inhibitors as a therapeutic strategy for stroke and neurodegenerative disease.Curr Drug Targets. 2012 Feb;13(2):199-206. doi: 10.2174/138945012799201676. Curr Drug Targets. 2012. PMID: 22204319 Review.
Cited by
-
Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome?Urol Res. 2012 Apr;40(2):95-112. doi: 10.1007/s00240-011-0448-9. Epub 2012 Jan 4. Urol Res. 2012. PMID: 22213019 Free PMC article. Review.
-
The role of reactive oxygen species derived from different NADPH oxidase isoforms and mitochondria in oxalate-induced oxidative stress and cell injury.Urolithiasis. 2022 Apr;50(2):149-158. doi: 10.1007/s00240-022-01309-2. Epub 2022 Feb 6. Urolithiasis. 2022. PMID: 35128564 Free PMC article.
-
Proposal for pathogenesis-based treatment options to reduce calcium oxalate stone recurrence.Asian J Urol. 2023 Jul;10(3):246-257. doi: 10.1016/j.ajur.2023.01.008. Epub 2023 Apr 13. Asian J Urol. 2023. PMID: 37538166 Free PMC article. Review.
-
Transcriptional study of hyperoxaluria and calcium oxalate nephrolithiasis in male rats: Inflammatory changes are mainly associated with crystal deposition.PLoS One. 2017 Nov 1;12(11):e0185009. doi: 10.1371/journal.pone.0185009. eCollection 2017. PLoS One. 2017. PMID: 29091707 Free PMC article.
-
Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis.Transl Androl Urol. 2014 Sep 1;3(3):256-276. doi: 10.3978/j.issn.2223-4683.2014.06.04. Transl Androl Urol. 2014. PMID: 25383321 Free PMC article.
References
-
- Khan SR. Renal tubular damage/dysfunction: key to the formation of kidney stones. Urol Res. 2006;34:86–91. - PubMed
-
- Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res. 2005;33:349–357. - PubMed
-
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. - PubMed
-
- Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–1607. - PubMed
-
- Shiose A, Kuroda J, Tsuruya K, et al. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

