The cell biology of polycystic kidney disease
- PMID: 21079243
- PMCID: PMC2983067
- DOI: 10.1083/jcb.201006173
The cell biology of polycystic kidney disease
Abstract
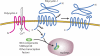
Polycystic kidney disease is a common genetic disorder in which fluid-filled cysts displace normal renal tubules. Here we focus on autosomal dominant polycystic kidney disease, which is attributable to mutations in the PKD1 and PKD2 genes and which is characterized by perturbations of renal epithelial cell growth control, fluid transport, and morphogenesis. The mechanisms that connect the underlying genetic defects to disease pathogenesis are poorly understood, but their exploration is shedding new light on interesting cell biological processes and suggesting novel therapeutic targets.
Figures

References
-
- Arnould T., Kim E., Tsiokas L., Jochimsen F., Grüning W., Chang J.D., Walz G. 1998. The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J. Biol. Chem. 273:6013–6018 10.1074/jbc.273.11.6013 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

