Cytoskeletal mechanics of proplatelet maturation and platelet release
- PMID: 21079248
- PMCID: PMC2983072
- DOI: 10.1083/jcb.201006102
Cytoskeletal mechanics of proplatelet maturation and platelet release
Abstract
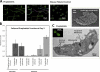
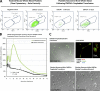
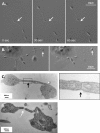
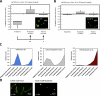
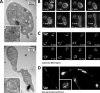
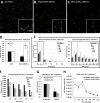
Megakaryocytes generate platelets by remodeling their cytoplasm into long proplatelet extensions, which serve as assembly lines for platelet production. Although the mechanics of proplatelet elongation have been studied, the terminal steps of proplatelet maturation and platelet release remain poorly understood. To elucidate this process, released proplatelets were isolated, and their conversion into individual platelets was assessed. This enabled us to (a) define and quantify the different stages in platelet maturation, (b) identify a new intermediate stage in platelet production, the preplatelet, (c) delineate the cytoskeletal mechanics involved in preplatelet/proplatelet interconversion, and (d) model proplatelet fission and platelet release. Preplatelets are anucleate discoid particles 2-10 µm across that have the capacity to convert reversibly into elongated proplatelets by twisting microtubule-based forces that can be visualized in proplatelets expressing GFP-β1-tubulin. The release of platelets from the ends of proplatelets occurs at an increasing rate in time during culture, as larger proplatelets undergo successive fission, and is potentiated by shear.
Figures


Comment in
-
Platelet precursors display bipolar behavior.J Cell Biol. 2010 Nov 15;191(4):699-700. doi: 10.1083/jcb.201010082. J Cell Biol. 2010. PMID: 21079242 Free PMC article.
References
-
- Behnke O., Forer A. 1998. From megakaryocytes to platelets: platelet morphogenesis takes place in the bloodstream. Eur. J. Haematol. Suppl. 61:3–23 - PubMed
-
- Choi E.S., Nichol J.L., Hokom M.M., Hornkohl A.C., Hunt P. 1995. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood. 85:402–413 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

