The implementation of isoniazid preventive therapy in HIV clinics: the experience from the TB/HIV in Rio (THRio) study
- PMID: 21079428
- PMCID: PMC3066070
- DOI: 10.1097/01.aids.0000391022.95412.a6
The implementation of isoniazid preventive therapy in HIV clinics: the experience from the TB/HIV in Rio (THRio) study
Abstract
Objective: The TB/HIV in Rio (THRio) study was launched in September 2005 to assess the impact of integrated tuberculosis (TB) and HIV treatment strategies in 29 HIV clinics in Rio de Janeiro, Brazil.
Design: THRio is a cluster-randomized trial (CRT) to determine whether routine screening for and treatment of latent TB in HIV clinic patients with access to antiretroviral therapy will reduce TB incidence at the clinic level. THRio is part of the Consortium to Respond Effectively to AIDS/TB Epidemic that is implementing research studies to assess the impact of bold, new public health paradigms for controlling the AIDS/TB epidemic.
Methods: Twenty-nine public primary HIV clinics were randomly assigned a date to begin implementing TB screening procedures and provision of isoniazid preventive therapy (IPT) for TB/HIV coinfected patients. Final analysis of the CRT is expected in 2011.
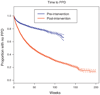
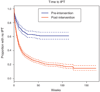
Results: Starting at date of tuberculin skin test (TST)/IPT implementation at each clinic through August 2010, 1670 HIV-infected patients initiated IPT, of which 215 are still receiving treatment. Of the remaining 1455 patients, 1230 (85%) completed therapy and only 20 (1.2%) patients initiating IPT reported adverse reactions leading to discontinuation of therapy. IPT completion was higher among HIV-infected patients receiving HAART (87%) than those not yet receiving HAART (79%, P < 0.01). Times to TST and IPT have markedly decreased postintervention, but remain considerably long. The richness of the THRio database has resulted in several analyses of this expansive cohort of HIV-infected patients that are reviewed here.
Conclusions: The national implementation of TST and IPT for HIV-positive patients in Brazil has been invigorated partly due to THRio's baseline results. Expanded use of IPT in HIV patients in Rio de Janeiro is achievable with high adherence and low adverse events, although this effort requires a package of activities including training, advocacy and reorganization of services.
Conflict of interest statement
Conflicts of interest: None.
Figures
References
-
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. - PubMed
-
- Santoro-Lopes G, de Pinho AM, Harrison LH, Schechter M. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis. 2002;34:543–546. - PubMed
-
- Jones JL, Hanson DL, Dworkin MS, DeCock KM Adult/Adolescent Spectrum of HIV Disease Group. HIV-associated tuberculosis in the era of highly active antiretroviral therapy. The Adult/Adolescent Spectrum of HIV Disease Group. Int J Tuberc Lung Dis. 2000;4:1026–1031. - PubMed
-
- Girardi E, Antonucci G, Vanacore P, Libanore M, Errante I, Matteeli A, et al. Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS. 2000;14:1985–1991. - PubMed
-
- Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

