Urinary cell levels of mRNA for OX40, OX40L, PD-1, PD-L1, or PD-L2 and acute rejection of human renal allografts
- PMID: 21079547
- PMCID: PMC3033230
- DOI: 10.1097/TP.0b013e3181ffbadd
Urinary cell levels of mRNA for OX40, OX40L, PD-1, PD-L1, or PD-L2 and acute rejection of human renal allografts
Abstract
Background: The positive costimulatory proteins OX40 and OX40L and negative regulatory proteins programmed death (PD)-1, PD ligand 1, and PD ligand 2 have emerged as significant regulators of acute rejection in experimental transplantation models.
Methods: We obtained 21 urine specimens from 21 renal allograft recipients with graft dysfunction and biopsy-confirmed acute rejection and 25 specimens from 25 recipients with stable graft function and normal biopsy results (stable). Urinary cell levels of mRNAs were measured using real-time quantitative polymerase chain reaction assays, and the levels were correlated with allograft status and outcomes.
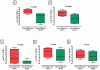
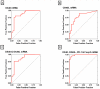
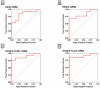
Results: Levels of OX40 mRNA (P<0.0001, Mann-Whitney test), OX40L mRNA (P=0.0004), and PD-1 mRNA (P=0.004), but not the mRNA levels of PD ligand 1 (P=0.08) or PD ligand 2 (P=0.20), were significantly higher in the urinary cells from the acute rejection group than the stable group. Receiver operating characteristic curve analysis demonstrated that acute rejection is predicted with a sensitivity of 95% and a specificity of 92% (area under the curve=0.98, 95% confidence interval 0.96-1.0, P<0.0001) using a combination of levels of mRNA for OX40, OX40L, PD-1, and levels of mRNA for the previously identified biomarker Foxp3. Within the acute rejection group, levels of mRNA for OX40 (P=0.0002), OX40L (P=0.0004), and Foxp3 (P=0.04) predicted acute rejection reversal, whereas only OX40 mRNA levels (P=0.04) predicted graft loss after acute rejection.
Conclusion: A linear combination of urinary cell levels of mRNA for OX40, OX40L, PD-1, and Foxp3 was a strong predictor of acute rejection in human renal allograft biopsies. This prediction model should be validated using an independent cohort of renal allograft recipients.
Figures


References
-
- Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342(9):605. - PubMed
-
- Bustami RT, Ojo AO, Wolfe RA, et al. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant. 2004;4(1):87. - PubMed
-
- Jamil B, Nicholls K, Becker GJ, Walker RG. Impact of acute rejection therapy on infections and malignancies in renal transplant recipients. Transplantation. 1999;68(10):1597–603. - PubMed
-
- Cornell LD, Smith RN, Colvin RB. Kidney transplantation: mechanisms of rejection and acceptance. Annu Rev Pathol. 2008;3:189. - PubMed
-
- Rothstein DM, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance. Immunol Rev. 2003;196:85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

