Catalytic asymmetric synthesis of both enantiomers of 4‑substituted 1,4-dihydropyridines with the use of bifunctional thiourea-ammonium salts bearing different counterions
- PMID: 21079568
- PMCID: PMC6259149
- DOI: 10.3390/molecules15118305
Catalytic asymmetric synthesis of both enantiomers of 4‑substituted 1,4-dihydropyridines with the use of bifunctional thiourea-ammonium salts bearing different counterions
Abstract
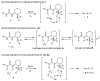
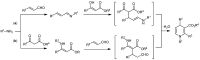
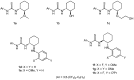
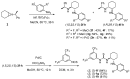
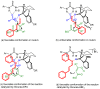
Organoammonium salts composed of a Brønsted acid and an anilinothiourea promoted the Michael addition of ß-keto esters and α,ß-unsaturated aldehydes in the presence of primary amines to give functionalized 1,4-dihydropyridines enantioselectively. With the use of the different Brønsted acids such as DFA and HBF(4) with the same bifunctional thiourea, both enantiomers of 4-substituted 1,4-dihydropyridine were synthesized from the same starting materials.
Figures
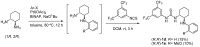
References
-
- Shibasaki M., Sasai H., Arai T. Asymmetric Catalysis with Heterobimet allic Compounds. Angew. Chem., Int. Ed. 1997;36:1236–1256. doi: 10.1002/anie.199712361. - DOI
-
- Wang Y., Deng L. Asymmetric Acid-Base Bifunctional Catalysis with Organic Molecules. In: Ojima I., editor. Catalytic Asymmetric Synthesis. 3rd. John Wiley & Sons; Hoboken, NJ, USA: 2010. pp. 59–94.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

