Role of NF-κB in the skeleton
- PMID: 21079651
- PMCID: PMC3193402
- DOI: 10.1038/cr.2010.159
Role of NF-κB in the skeleton
Abstract
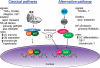
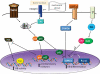
Since the discovery that deletion of the NF-κB subunits p50 and p52 causes osteopetrosis in mice, there has been considerable interest in the role of NF-κB signaling in bone. NF-κB controls the differentiation or activity of the major skeletal cell types - osteoclasts, osteoblasts, osteocytes and chondrocytes. However, with five NF-κB subunits and two distinct activation pathways, not all NF-κB signals lead to the same physiologic responses. In this review, we will describe the roles of various NF-κB proteins in basal bone homeostasis and disease states, and explore how NF-κB inhibition might be utilized therapeutically.
Figures

References
-
- Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med. 1997;3:1285–1289. - PubMed
-
- Grigoriadis AE, Wang Z-Q, Cecchini MG, et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

