Conflict between noise and plasticity in yeast
- PMID: 21079670
- PMCID: PMC2973811
- DOI: 10.1371/journal.pgen.1001185
Conflict between noise and plasticity in yeast
Abstract
Gene expression responds to changes in conditions but also stochastically among individuals. In budding yeast, both expression responsiveness across conditions ("plasticity") and cell-to-cell variation ("noise") have been quantified for thousands of genes and found to correlate across genes. It has been argued therefore that noise and plasticity may be strongly coupled and mechanistically linked. This is consistent with some theoretical ideas, but a strong coupling between noise and plasticity also has the potential to introduce cost-benefit conflicts during evolution. For example, if high plasticity is beneficial (genes need to respond to the environment), but noise is detrimental (fluctuations are harmful), then strong coupling should be disfavored. Here, evidence is presented that cost-benefit conflicts do occur and that they constrain the evolution of gene expression and promoter usage. In contrast to recent assertions, coupling between noise and plasticity is not a general property, but one associated with particular mechanisms of transcription initiation. Further, promoter architectures associated with coupling are avoided when noise is most likely to be detrimental, and noise and plasticity are largely independent traits for core cellular components. In contrast, when genes are duplicated noise-plasticity coupling increases, consistent with reduced detrimental affects of expression variation. Noise-plasticity coupling is, therefore, an evolvable trait that may constrain the emergence of highly responsive gene expression and be selected against during evolution. Further, the global quantitative data in yeast suggest that one mechanism that relieves the constraints imposed by noise-plasticity coupling is gene duplication, providing an example of how duplication can facilitate escape from adaptive conflicts.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
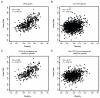

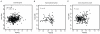

Comment in
-
Noise versus plasticity.Nat Rev Genet. 2011 Jan;12(1):4. doi: 10.1038/nrg2925. Epub 2010 Nov 30. Nat Rev Genet. 2011. PMID: 21116308 No abstract available.
References
-
- Ihmels J, Friedlander G, Bergmann S, Sarig O, Ziv Y, et al. Revealing modular organization in the yeast transcriptional network. Nat Genet. 2002;31:370–377. - PubMed
-
- Newman JR, Ghaemmaghami S, Ihmels J, Breslow DK, Noble M, et al. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature. 2006;441:840–846. - PubMed
-
- Choi JK, Kim YJ. Intrinsic variability of gene expression encoded in nucleosome positioning sequences. Nat Genet. 2009;41:498–503. - PubMed
-
- Landry CR, Lemos B, Rifkin SA, Dickinson WJ, Hartl DL. Genetic properties influencing the evolvability of gene expression. Science. 2007;317:118–121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

