HTLV-1 evades type I interferon antiviral signaling by inducing the suppressor of cytokine signaling 1 (SOCS1)
- PMID: 21079688
- PMCID: PMC2973829
- DOI: 10.1371/journal.ppat.1001177
HTLV-1 evades type I interferon antiviral signaling by inducing the suppressor of cytokine signaling 1 (SOCS1)
Abstract
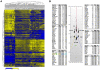
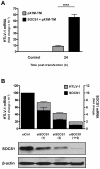
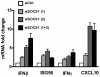
Human T cell leukemia virus type 1 (HTLV-1) is the etiologic agent of Adult T cell Leukemia (ATL) and the neurological disorder HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Although the majority of HTLV-1-infected individuals remain asymptomatic carriers (AC) during their lifetime, 2-5% will develop either ATL or HAM/TSP, but never both. To better understand the gene expression changes in HTLV-1-associated diseases, we examined the mRNA profiles of CD4+ T cells isolated from 7 ATL, 12 HAM/TSP, 11 AC and 8 non-infected controls. Using genomic approaches followed by bioinformatic analysis, we identified gene expression pattern characteristic of HTLV-1 infected individuals and particular disease states. Of particular interest, the suppressor of cytokine signaling 1--SOCS1--was upregulated in HAM/TSP and AC patients but not in ATL. Moreover, SOCS1 was positively correlated with the expression of HTLV-1 mRNA in HAM/TSP patient samples. In primary PBMCs transfected with a HTLV-1 proviral clone and in HTLV-1-transformed MT-2 cells, HTLV-1 replication correlated with induction of SOCS1 and inhibition of IFN-α/β and IFN-stimulated gene expression. Targeting SOCS1 with siRNA restored type I IFN production and reduced HTLV-1 replication in MT-2 cells. Conversely, exogenous expression of SOCS1 resulted in enhanced HTLV-1 mRNA synthesis. In addition to inhibiting signaling downstream of the IFN receptor, SOCS1 inhibited IFN-β production by targeting IRF3 for ubiquitination and proteasomal degradation. These observations identify a novel SOCS1 driven mechanism of evasion of the type I IFN antiviral response against HTLV-1.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

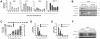

Similar articles
-
The IL-18, IL-12, and IFN-γ expression in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients, HTLV-1 carriers, and healthy subjects.J Neurovirol. 2020 Jun;26(3):338-346. doi: 10.1007/s13365-020-00832-5. Epub 2020 Apr 8. J Neurovirol. 2020. PMID: 32270468
-
Blockade of IL-2 receptor suppresses HTLV-I and IFN-gamma expression in patients with HTLV-I-associated myelopathy/tropical spastic paraparesis.Intern Med. 2007;46(7):347-51. doi: 10.2169/internalmedicine.46.6118. Epub 2007 Apr 2. Intern Med. 2007. PMID: 17409595
-
Tax secretion from peripheral blood mononuclear cells and Tax detection in plasma of patients with human T-lymphotropic virus-type 1-associated myelopathy/tropical spastic paraparesis and asymptomatic carriers.J Med Virol. 2016 Mar;88(3):521-31. doi: 10.1002/jmv.24342. Epub 2015 Oct 19. J Med Virol. 2016. PMID: 26241614
-
Immunopathogenesis of HTLV-1-assoaciated myelopathy/tropical spastic paraparesis (HAM/TSP).Life Sci. 2014 May 28;104(1-2):9-14. doi: 10.1016/j.lfs.2014.03.025. Epub 2014 Apr 3. Life Sci. 2014. PMID: 24704970 Review.
-
Tropical spastic paraparesis and HTLV-1 associated myelopathy: clinical, epidemiological, virological and therapeutic aspects.Rev Neurol (Paris). 2012 Mar;168(3):257-69. doi: 10.1016/j.neurol.2011.12.006. Epub 2012 Mar 7. Rev Neurol (Paris). 2012. PMID: 22405461 Review.
Cited by
-
Human T cell leukemia virus type 1 Tax inhibits innate antiviral signaling via NF-kappaB-dependent induction of SOCS1.J Virol. 2011 Jul;85(14):6955-62. doi: 10.1128/JVI.00007-11. Epub 2011 May 18. J Virol. 2011. PMID: 21593151 Free PMC article.
-
Systems biology approaches reveal a specific interferon-inducible signature in HTLV-1 associated myelopathy.PLoS Pathog. 2012 Jan;8(1):e1002480. doi: 10.1371/journal.ppat.1002480. Epub 2012 Jan 26. PLoS Pathog. 2012. PMID: 22291590 Free PMC article. Clinical Trial.
-
Human endogenous retroviruses and the nervous system.Handb Clin Neurol. 2014;123:465-85. doi: 10.1016/B978-0-444-53488-0.00022-5. Handb Clin Neurol. 2014. PMID: 25015500 Free PMC article. Review. No abstract available.
-
Human T-lymphotropic virus proteins and post-translational modification pathways.World J Virol. 2012 Aug 12;1(4):115-30. doi: 10.5501/wjv.v1.i4.115. World J Virol. 2012. PMID: 24175216 Free PMC article. Review.
-
Prognoses of patients with acute-on-chronic hepatitis B liver failure are closely associated with altered SOCS1 mRNA expression and cytokine production following glucocorticoid treatment.Cell Mol Immunol. 2014 Jul;11(4):396-404. doi: 10.1038/cmi.2014.23. Epub 2014 Apr 14. Cell Mol Immunol. 2014. PMID: 24727541 Free PMC article.
References
-
- Grant C, Barmak K, Alefantis T, Yao J, Jacobson S, et al. Human T cell leukemia virus type I and neurologic disease: events in bone marrow, peripheral blood, and central nervous system during normal immune surveillance and neuroinflammation. J Cell Physiol. 2002;190:133–159. - PubMed
-
- Bangham CR, Meekings K, Toulza F, Nejmeddine M, Majorovits E, et al. The immune control of HTLV-1 infection: selection forces and dynamics. Front Biosci. 2009;14:2889–2903. - PubMed
-
- Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. - PubMed
-
- Sabouri AH, Usuku K, Hayashi D, Izumo S, Ohara Y, et al. Impaired function of human T-lymphotropic virus type 1 (HTLV-1)-specific CD8+ T cells in HTLV-1-associated neurologic disease. Blood. 2008;112:2411–2420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

