Inhibition of glioblastoma growth by the thiadiazolidinone compound TDZD-8
- PMID: 21079728
- PMCID: PMC2975629
- DOI: 10.1371/journal.pone.0013879
Inhibition of glioblastoma growth by the thiadiazolidinone compound TDZD-8
Abstract
Background: Thiadiazolidinones (TDZD) are small heterocyclic compounds first described as non-ATP competitive inhibitors of glycogen synthase kinase 3β (GSK-3β). In this study, we analyzed the effects of 4-benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione (TDZD-8), on murine GL261 cells growth in vitro and on the growth of established intracerebral murine gliomas in vivo.
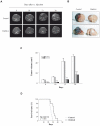
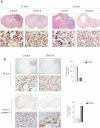
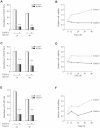
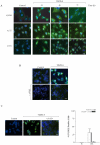
Methodology/principal findings: Our data show that TDZD-8 decreased proliferation and induced apoptosis of GL261 glioblastoma cells in vitro, delayed tumor growth in vivo, and augmented animal survival. These effects were associated with an early activation of extracellular signal-regulated kinase (ERK) pathway and increased expression of EGR-1 and p21 genes. Also, we observed a sustained activation of the ERK pathway, a concomitant phosphorylation and activation of ribosomal S6 kinase (p90RSK) and an inactivation of GSK-3β by phosphorylation at Ser 9. Finally, treatment of glioblastoma stem cells with TDZD-8 resulted in an inhibition of proliferation and self-renewal of these cells.
Conclusions/significance: Our results suggest that TDZD-8 uses a novel mechanism to target glioblastoma cells, and that malignant progenitor population could be a target of this compound.
Conflict of interest statement
Figures

References
-
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. - PubMed
-
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. - PubMed
-
- Fomchenko EI, Holland EC. Mouse models of brain tumors and their applications in preclinical trials. Clin Cancer Res. 2006;12:5288–5297. - PubMed
-
- Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. - PubMed
-
- Kim L, Kimmel AR. GSK3, a master switch regulating cell-fate specification and tumorigenesis. Curr Opin Genet Dev. 2000;10:508–514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

