Proteomic analysis of the dysferlin protein complex unveils its importance for sarcolemmal maintenance and integrity
- PMID: 21079765
- PMCID: PMC2974636
- DOI: 10.1371/journal.pone.0013854
Proteomic analysis of the dysferlin protein complex unveils its importance for sarcolemmal maintenance and integrity
Abstract
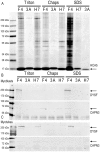
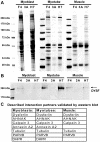
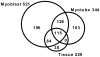
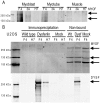
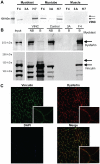
Dysferlin is critical for repair of muscle membranes after damage. Mutations in dysferlin lead to a progressive muscular dystrophy. Recent studies suggest additional roles for dysferlin. We set out to study dysferlin's protein-protein interactions to obtain comprehensive knowledge of dysferlin functionalities in a myogenic context. We developed a robust and reproducible method to isolate dysferlin protein complexes from cells and tissue. We analyzed the composition of these complexes in cultured myoblasts, myotubes and skeletal muscle tissue by mass spectrometry and subsequently inferred potential protein functions through bioinformatics analyses. Our data confirm previously reported interactions and support a function for dysferlin as a vesicle trafficking protein. In addition novel potential functionalities were uncovered, including phagocytosis and focal adhesion. Our data reveal that the dysferlin protein complex has a dynamic composition as a function of myogenic differentiation. We provide additional experimental evidence and show dysferlin localization to, and interaction with the focal adhesion protein vinculin at the sarcolemma. Finally, our studies reveal evidence for cross-talk between dysferlin and its protein family member myoferlin. Together our analyses show that dysferlin is not only a membrane repair protein but also important for muscle membrane maintenance and integrity.
Conflict of interest statement
Figures

References
-
- Anderson LV, Davison K, Moss JA, Young C, Cullen MJ, et al. Dysferlin is a plasma membrane protein and is expressed early in human development. Hum Mol Genet. 1999;8:855–861. ddc087 [pii] - PubMed
-
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, et al. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. 10.1038/nature01573 [doi];nature01573 [pii] - PubMed
-
- Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, et al. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998;20:37–42. 10.1038/1689 [doi] - PubMed
-
- Illa I, Serrano-Munuera C, Gallardo E, Lasa A, Rojas-Garcia R, et al. Distal anterior compartment myopathy: a dysferlin mutation causing a new muscular dystrophy phenotype. Ann Neurol. 2001;49:130–134. - PubMed
-
- Liu J, Aoki M, Illa I, Wu C, Fardeau M, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet. 1998;20:31–36. 10.1038/1682 [doi] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

