Changes in hepatic gene expression upon oral administration of taurine-conjugated ursodeoxycholic acid in ob/ob mice
- PMID: 21079772
- PMCID: PMC2974643
- DOI: 10.1371/journal.pone.0013858
Changes in hepatic gene expression upon oral administration of taurine-conjugated ursodeoxycholic acid in ob/ob mice
Abstract
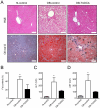
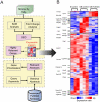
Nonalcoholic fatty liver disease (NAFLD) is highly prevalent and associated with considerable morbidities. Unfortunately, there is no currently available drug established to treat NAFLD. It was recently reported that intraperitoneal administration of taurine-conjugated ursodeoxycholic acid (TUDCA) improved hepatic steatosis in ob/ob mice. We hereby examined the effect of oral TUDCA treatment on hepatic steatosis and associated changes in hepatic gene expression in ob/ob mice. We administered TUDCA to ob/ob mice at a dose of 500 mg/kg twice a day by gastric gavage for 3 weeks. Body weight, glucose homeostasis, endoplasmic reticulum (ER) stress, and hepatic gene expression were examined in comparison with control ob/ob mice and normal littermate C57BL/6J mice. Compared to the control ob/ob mice, TUDCA treated ob/ob mice revealed markedly reduced liver fat stained by oil red O (44.2±5.8% vs. 21.1±10.4%, P<0.05), whereas there was no difference in body weight, oral glucose tolerance, insulin sensitivity, and ER stress. Microarray analysis of hepatic gene expression demonstrated that oral TUDCA treatment mainly decreased the expression of genes involved in de novo lipogenesis among the components of lipid homeostasis. At pathway levels, oral TUDCA altered the genes regulating amino acid, carbohydrate, and drug metabolism in addition to lipid metabolism. In summary, oral TUDCA treatment decreased hepatic steatosis in ob/ob mice by cooperative regulation of multiple metabolic pathways, particularly by reducing the expression of genes known to regulate de novo lipogenesis.
Conflict of interest statement
Figures




References
-
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. - PubMed
-
- Greenfield V, Cheung O, Sanyal AJ. Recent advances in nonalcholic fatty liver disease. Curr Opin Gastroenterol. 2008;24:320–327. - PubMed
-
- Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939–960. - PubMed
-
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. - PubMed
-
- Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–2121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

