ER-α36, a novel variant of ER-α, mediates estrogen-stimulated proliferation of endometrial carcinoma cells via the PKCδ/ERK pathway
- PMID: 21079811
- PMCID: PMC2973969
- DOI: 10.1371/journal.pone.0015408
ER-α36, a novel variant of ER-α, mediates estrogen-stimulated proliferation of endometrial carcinoma cells via the PKCδ/ERK pathway
Abstract
Background: Recently, a variant of ER-α, ER-α36 was identified and cloned. ER-α36 lacks intrinsic transcription activity and mainly mediates non-genomic estrogen signaling. The purpose of this study was to investigate the function and the underlying mechanisms of ER-α36 in growth regulation of endometrial Ishikawa cancer cells.
Methods: The cellular localization of ER-α36 and ER-α66 were determined by immunofluorescence in the Ishikawa cells. Ishikawa endometrial cancer control cells transfected with an empty expression vector, Ishikawa cells with shRNA knockdown of ER-α36 (Ishikawa/RNAiER36) and Ishikawa cells with shRNA knockdown of ER-α66 (Ishikawa/RNAiER66) were treated with E2 and E2-conjugated to bovine serum albumin (E2-BSA, membrane impermeable) in the absence and presence of different kinase inhibitors HBDDE, bisindolylmaleimide, rottlerin, H89 and U0126. The phosphorylation levels of signaling molecules and cyclin D1/cdk4 expression were examined with Western blot analysis and cell growth was monitored with the MTT assay.
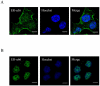
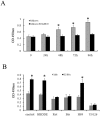
Results: Immunofluorescence staining of Ishikawa cells demonstrated that ER-α36 was expressed mainly on the plasma membrane and in the cytoplasm, while ER-α66 was predominantly localized in the cell nucleus. Both E2 and E2-BSA rapidly activated PKCδ not PKCα in Ishikawa cells, which could be abrogated by ER-α36 shRNA expression. E2-and E2-BSA-induced ERK phosphorylation required ER-α36 and PKCδ. However, only E2 was able to induce Camp-dependent protein kinase A (PKA) phosphorylation. Furthermore, E2 enhances cyclin D1/cdk4 expression via ER-α36.
Conclusion: E2 activates the PKCδ/ERK pathway and enhances cyclin D1/cdk4 expression via the membrane-initiated signaling pathways mediated by ER-α36, suggesting a possible involvement of ER-α36 in E2-dependent growth-promoting effects in endometrial cancer cells.
Conflict of interest statement
Figures




References
-
- Shang Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat Rev Cancer. 2006;6:360–368. - PubMed
-
- Chaudhry P, Asselin E. Resistance to chemotherapy and hormone therapy in endometrial cancer. Endocr Relat Cancer. 2009;16:363–380. - PubMed
-
- Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. - PubMed
-
- Muramatsu M, Inoue S. Estrogen receptors: how do they control reproductive and nonreproductive functions? Biochem Biophys Res Commun. 2000;270:1–10. - PubMed
-
- Zhang Y, Wei L, Wang J, Sun T. Role of phosphatase PTEN in the activation of extracellular signal-regulated kinases induced by estradiol in endometrial carcinoma cells. Chin Med J (Engl) 2003;116:383–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

