Modulation of calcium channels by taurine acting via a metabotropic-like glycine receptor
- PMID: 21080059
- PMCID: PMC11498753
- DOI: 10.1007/s10571-010-9574-0
Modulation of calcium channels by taurine acting via a metabotropic-like glycine receptor
Abstract
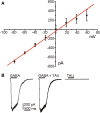
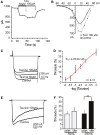
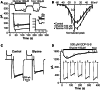
Taurine is one of the most abundant free amino acids in the central nervous system, where it displays several functions. However, its molecular targets remain unknown. It is well known that taurine can activate GABA-A and strychnine-sensitive glycine receptors, which increases a chloride conductance. In this study, we describe that acute application of taurine induces a dose-dependent inhibition of voltage-dependent calcium channels in chromaffin cells from bovine adrenal medullae. This taurine effect was not explained by the activation of either GABA-A, GABA-B or strychnine-sensitive glycine receptors. Interestingly, glycine mimicked the modulatory action exerted by taurine on calcium channels, although the acute application of glycine did not elicit any ionic current in these cells. Additionally, the modulation of calcium channels exerted by both taurine and glycine was prevented by the intracellular dialysis of GDP-β-S. Thus, the modulation of voltage-dependent calcium channels by taurine seems to be mediated by a metabotropic-like glycinergic receptor coupled to G-protein activation in a membrane delimited pathway.
Figures

Similar articles
-
Characterization of strychnine-sensitive glycine receptors in acutely isolated adult rat basolateral amygdala neurons.Brain Res. 2000 Mar 24;859(2):341-51. doi: 10.1016/s0006-8993(00)02026-6. Brain Res. 2000. PMID: 10719083
-
Taurine and the control of basal hormone release from rat neurohypophysis.Exp Neurol. 2003 Oct;183(2):330-7. doi: 10.1016/s0014-4886(03)00105-5. Exp Neurol. 2003. PMID: 14552874
-
Reciprocal regulation between taurine and glutamate response via Ca2+-dependent pathways in retinal third-order neurons.J Biomed Sci. 2010 Aug 24;17 Suppl 1(Suppl 1):S5. doi: 10.1186/1423-0127-17-S1-S5. J Biomed Sci. 2010. PMID: 20804625 Free PMC article.
-
Direct and remote modulation of L-channels in chromaffin cells: distinct actions on alpha1C and alpha1D subunits?Mol Neurobiol. 2004 Feb;29(1):73-96. doi: 10.1385/MN:29:1:73. Mol Neurobiol. 2004. PMID: 15034224 Review.
-
Need rods? Get glycine receptors and taurine.Neuron. 2004 Mar 25;41(6):839-41. doi: 10.1016/s0896-6273(04)00153-9. Neuron. 2004. PMID: 15046714 Review.
Cited by
-
Orphan receptor GPR158 serves as a metabotropic glycine receptor: mGlyR.Science. 2023 Mar 31;379(6639):1352-1358. doi: 10.1126/science.add7150. Epub 2023 Mar 30. Science. 2023. PMID: 36996198 Free PMC article.
-
Reply to letter of Peter Good.Neuropsychol Rev. 2011 Mar;21(1):70-1. doi: 10.1007/s11065-011-9159-9. Epub 2011 Jan 19. Neuropsychol Rev. 2011. PMID: 21246288 No abstract available.
References
-
- Castro E, Gonzalez MA, Oset-Gasque MJ (2003) Distribution of γ-aminobutyric acid receptors in cultured adrenergic and noradrenergic bovine chromaffin cells. J Neurosci Res 71:375–382 - PubMed
-
- del Olmo N, Bustamante J, Martín del Rio R, Solís JM (2000) Taurine activates GABAA but not GABAB receptors in rat hippocampal CA1 area. Brain Res 864:298–307 - PubMed
-
- del Olmo N, Suárez LM, Orensanz LM, Suárez F, Bustamante J, Duarte JM, Martín del Río R, Solís JM (2004) Role of taurine uptake on the induction of long-term synaptic potentiation. Eur J Neurosci 19:1875–1886 - PubMed
-
- El Idrissi A (2008) Taurine increases mitochondrial buffering of calcium: role in neuroprotection. Amino Acids 34:321–328 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

