A long peptide from MELOE-1 contains multiple HLA class II T cell epitopes in addition to the HLA-A*0201 epitope: an attractive candidate for melanoma vaccination
- PMID: 21080167
- PMCID: PMC11029773
- DOI: 10.1007/s00262-010-0938-6
A long peptide from MELOE-1 contains multiple HLA class II T cell epitopes in addition to the HLA-A*0201 epitope: an attractive candidate for melanoma vaccination
Abstract
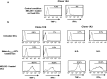
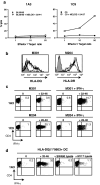
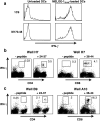
CD4(+) T cells contribute importantly to the antitumor T cell response, and thus, long peptides comprising CD4 and CD8 epitopes may be efficient cancer vaccines. We have previously identified an overexpressed antigen in melanoma, MELOE-1, presenting a CD8(+) T cell epitope, MELOE-1(36-44), in the HLA-A*0201 context. A T cell repertoire against this epitope is present in HLA-A*0201+ healthy subjects and melanoma patients and the adjuvant injection of TIL containing MELOE-1 specific CD8(+) T cells to melanoma patients was shown to be beneficial. In this study, we looked for CD4(+) T cell epitopes in the vicinity of the HLA-A*0201 epitope. Stimulation of PBMC from healthy subjects with MELOE-1(26-46) revealed CD4 responses in multiple HLA contexts and by cloning responsive CD4(+) T cells, we identified one HLA-DRβ1*1101-restricted and one HLA-DQβ1*0603-restricted epitope. We showed that the two epitopes could be efficiently presented to CD4(+) T cells by MELOE-1-loaded dendritic cells but not by MELOE-1+ melanoma cell-lines. Finally, we showed that the long peptide MELOE-1(22-46), containing the two optimal class II epitopes and the HLA-A*0201 epitope, was efficiently processed by DC to stimulate CD4(+) and CD8(+) T cell responses in vitro, making it a potential candidate for melanoma vaccination.
Figures


Similar articles
-
MELOE-1 antigen contains multiple HLA class II T cell epitopes recognized by Th1 CD4+ T cells from melanoma patients.PLoS One. 2012;7(12):e51716. doi: 10.1371/journal.pone.0051716. Epub 2012 Dec 20. PLoS One. 2012. PMID: 23284752 Free PMC article.
-
Frequent occurrence of high affinity T cells against MELOE-1 makes this antigen an attractive target for melanoma immunotherapy.Eur J Immunol. 2010 Jun;40(6):1786-94. doi: 10.1002/eji.200940132. Eur J Immunol. 2010. PMID: 20217862
-
Melan-A/MART-1-specific CD4 T cells in melanoma patients: identification of new epitopes and ex vivo visualization of specific T cells by MHC class II tetramers.J Immunol. 2006 Nov 15;177(10):6769-79. doi: 10.4049/jimmunol.177.10.6769. J Immunol. 2006. PMID: 17082590
-
Melanoma antigens recognised by CD8+ and CD4+ T cells.Forum (Genova). 2000 Jul-Sep;10(3):256-70. Forum (Genova). 2000. PMID: 11007933 Review.
-
The use of HLA class I tetramers to design a vaccination strategy for melanoma patients.Immunol Rev. 2002 Oct;188:155-63. doi: 10.1034/j.1600-065x.2002.18814.x. Immunol Rev. 2002. PMID: 12445289 Review.
Cited by
-
IRES-dependent translation of the long non coding RNA meloe in melanoma cells produces the most immunogenic MELOE antigens.Oncotarget. 2016 Sep 13;7(37):59704-59713. doi: 10.18632/oncotarget.10923. Oncotarget. 2016. PMID: 27486971 Free PMC article.
-
Heteroclitic XBP1 peptides evoke tumor-specific memory cytotoxic T lymphocytes against breast cancer, colon cancer, and pancreatic cancer cells.Oncoimmunology. 2014 Dec 2;3(12):e970914. doi: 10.4161/21624011.2014.970914. eCollection 2014. Oncoimmunology. 2014. PMID: 25941601 Free PMC article.
-
Therapeutic Cancer Vaccines-Antigen Discovery and Adjuvant Delivery Platforms.Pharmaceutics. 2022 Jul 11;14(7):1448. doi: 10.3390/pharmaceutics14071448. Pharmaceutics. 2022. PMID: 35890342 Free PMC article. Review.
-
Database of T cell-defined human tumor antigens: the 2013 update.Cancer Immun. 2013 Jul 15;13:15. Print 2013. Cancer Immun. 2013. PMID: 23882160 Free PMC article. Review.
-
MELOE-1 antigen contains multiple HLA class II T cell epitopes recognized by Th1 CD4+ T cells from melanoma patients.PLoS One. 2012;7(12):e51716. doi: 10.1371/journal.pone.0051716. Epub 2012 Dec 20. PLoS One. 2012. PMID: 23284752 Free PMC article.
References
-
- Fayolle C, Deriaud E, Leclerc C. In vivo induction of cytotoxic t cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol. 1991;147:4069–4073. - PubMed
-
- Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179:5033–5040. - PubMed
-
- Gritzapis AD, Voutsas IF, Lekka E, Papamichail M, Baxevanis CN. Peptide vaccination breaks tolerance to HER-2/neu by generating vaccine-specific fasL+ CD4+ T cells: first evidence for intratumor apoptotic regulatory T cells. Cancer Res. 2010;70:2686–2696. doi: 10.1158/0008-5472.CAN-09-2517. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

