Bioassay-directed fractionation for discovery of bioactive neutral lipids guided by relative mass defect filtering and multiplexed collision-induced dissociation
- PMID: 21080510
- PMCID: PMC4019978
- DOI: 10.1002/rcm.4796
Bioassay-directed fractionation for discovery of bioactive neutral lipids guided by relative mass defect filtering and multiplexed collision-induced dissociation
Abstract
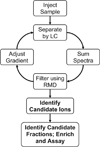
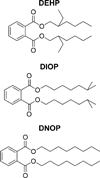
We report a synergistic method using bioassay-directed liquid chromatography fractionation and time-of-flight mass spectrometry to guide and accelerate bioactive compound discovery. To steer purification and assays toward anticipated neutral lipid activators of a constitutive androstane receptor splice variant, a relative mass defect filter was calculated, based on the ratio of the mass defect to the measured ion mass, and used to reduce the number of candidate ion masses. Mass measurements often lack sufficient accuracy to provide unambiguous assignments of elemental compositions, and since the relative mass defect reflects fractional hydrogen content of ions, this value is largely determined by the hydrogen content of a compound's biosynthetic precursors. A relative mass defect window ranging from 600-1000 ppm, consistent with an assortment of lipids, was chosen to assess the number of candidate ions in fractions of fetal bovine serum. This filter reduced the number of candidate ion m/z values from 1345 to 892, which was further reduced to 21 by intensity and isotope filtering. Accurate mass measurements from time-of-flight mass spectrometry and fragment ion masses generated using nonselective collision-induced dissociation suggested dioctyl phthalate as one of few neutral lipid constituents in the active fraction. The identity of this compound was determined to be di(2-ethylhexyl) phthalate using GC/MS, and it was ranked as a promising candidate for reporter assay screening.
Copyright © 2010 John Wiley & Sons, Ltd.
Figures



Similar articles
-
Analysis of di-n-butylphthalate biotransformation in cattle by liquid chromatography/ion trap mass spectrometry/mass spectrometry.J Mass Spectrom. 1998 Sep;33(9):803-10. doi: 10.1002/(SICI)1096-9888(199809)33:9<803::AID-JMS689>3.0.CO;2-0. J Mass Spectrom. 1998. PMID: 9768498
-
Method for the elucidation of the elemental composition of low molecular mass chemicals using exact masses of product ions and neutral losses: application to environmental chemicals measured by liquid chromatography with hybrid quadrupole/time-of-flight mass spectrometry.Rapid Commun Mass Spectrom. 2005;19(23):3500-16. doi: 10.1002/rcm.2220. Rapid Commun Mass Spectrom. 2005. PMID: 16261657
-
Characterization of the designer drug bk-2C-B (2-amino-1-(bromo-dimethoxyphenyl)ethan-1-one) by gas chromatography/mass spectrometry without and with derivatization with 2,2,2-trichloroethyl chloroformate, liquid chromatography/high-resolution mass spectrometry, and nuclear magnetic resonance.Rapid Commun Mass Spectrom. 2015 Jul 15;29(13):1196-204. doi: 10.1002/rcm.7211. Rapid Commun Mass Spectrom. 2015. PMID: 26395784
-
Insight into chemical basis of traditional Chinese medicine based on the state-of-the-art techniques of liquid chromatography-mass spectrometry.Acta Pharm Sin B. 2021 Jun;11(6):1469-1492. doi: 10.1016/j.apsb.2021.02.017. Epub 2021 Feb 26. Acta Pharm Sin B. 2021. PMID: 34221863 Free PMC article. Review.
-
Role of liquid chromatography-high-resolution mass spectrometry (LC-HR/MS) in clinical toxicology.Clin Toxicol (Phila). 2012 Sep;50(8):733-42. doi: 10.3109/15563650.2012.713108. Epub 2012 Aug 13. Clin Toxicol (Phila). 2012. PMID: 22888997 Review.
Cited by
-
A Systematic Approach to Discover New Natural Product Scaffolds Using Database-Derived Relative Mass Spectral Defects and Molecular Networking.JACS Au. 2025 Jan 16;5(2):653-665. doi: 10.1021/jacsau.4c00889. eCollection 2025 Feb 24. JACS Au. 2025. PMID: 40017746 Free PMC article.
-
Water-soluble saponins accumulate in drought-stressed switchgrass and may inhibit yeast growth during bioethanol production.Biotechnol Biofuels Bioprod. 2022 Oct 31;15(1):116. doi: 10.1186/s13068-022-02213-y. Biotechnol Biofuels Bioprod. 2022. PMID: 36310161 Free PMC article.
-
Relative mass defect filtering of mass spectra: a path to discovery of plant specialized metabolites.Plant Physiol. 2015 Apr;167(4):1221-32. doi: 10.1104/pp.114.251165. Epub 2015 Feb 6. Plant Physiol. 2015. PMID: 25659383 Free PMC article.
-
Striking natural diversity in glandular trichome acylsugar composition is shaped by variation at the Acyltransferase2 locus in the wild tomato Solanum habrochaites.Plant Physiol. 2012 Dec;160(4):1854-70. doi: 10.1104/pp.112.204735. Epub 2012 Oct 9. Plant Physiol. 2012. PMID: 23054567 Free PMC article.
-
Trends in the application of high-resolution mass spectrometry for human biomonitoring: An analytical primer to studying the environmental chemical space of the human exposome.Environ Int. 2017 Mar;100:32-61. doi: 10.1016/j.envint.2016.11.026. Epub 2017 Jan 4. Environ Int. 2017. PMID: 28062070 Free PMC article. Review.
References
-
- DiMasi JA, Hansen RW, Grabowski HG, Lasagna L. J. Health Econ. 1991;10:107. - PubMed
-
- DiMasi JA, Hansen RW, Grabowski HG. J. Health Econ. 2003;22:151. - PubMed
-
- Fauchère J-L, Boutin JA, Henlin J-M, Kucharczyk N, Ortuno J-C. Chemom. Intell. Lab. Syst. 1998;43:43.
-
- Ertl P, Roggo S, Schuffenhauer A. J. Chem. Inf. Modeling. 2007;48:68. - PubMed
-
- Young SM, Curry MS, Ransom JT, Ballesteros JA, Prossnitz ER, Sklar LA, Edwards BS. J. Biomol. Screen. 2004;9:103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
