The Akt-inhibitor Erufosine induces apoptotic cell death in prostate cancer cells and increases the short term effects of ionizing radiation
- PMID: 21080918
- PMCID: PMC2998511
- DOI: 10.1186/1748-717X-5-108
The Akt-inhibitor Erufosine induces apoptotic cell death in prostate cancer cells and increases the short term effects of ionizing radiation
Abstract
Background and purpose: The phosphatidylinositol-3-kinase (PI3K)/Akt pathway is frequently deregulated in prostate cancer and associated with neoplastic transformation, malignant progression, and enhanced resistance to classical chemotherapy and radiotherapy. Thus, it is a promising target for therapeutic intervention. In the present study, the cytotoxic action of the Akt inhibitor Erufosine (ErPC3) was analyzed in prostate cancer cells and compared to the cytotoxicity of the PI3K inhibitor LY294002. Moreover, the efficacy of combined treatment with Akt inhibitors and ionizing radiation in prostate cancer cells was examined.
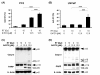
Materials and methods: Prostate cancer cell lines PC3, DU145, and LNCaP were treated with ErPC3 (1-100 µM), LY294002 (25-100 µM), irradiated (0-10 Gy), or subjected to combined treatments. Cell viability was determined by the WST-1 assay. Apoptosis induction was analyzed by flow cytometry after staining with propidium iodide in a hypotonic citrate buffer, and by Western blotting using antibodies against caspase-3 and its substrate PARP. Akt activity and regulation of the expression of Bcl-2 family members and key downstream effectors involved in apoptosis regulation were examined by Western blot analysis.
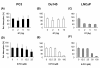
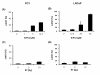
Results: The Akt inhibitor ErPC3 exerted anti-neoplastic effects in prostate cancer cells, however with different potency. The anti-neoplastic action of ErPC3 was associated with reduced phosphoserine 473-Akt levels and induction of apoptosis. PC3 and LNCaP prostate cancer cells were also sensitive to treatment with the PI3K inhibitor LY294002. However, the ErPC3-sensitive PC3-cells were less susceptible to LY294002 than the ErPC3-refractory LNCaP cells. Although both cell lines were largely resistant to radiation-induced apoptosis, both cell lines showed higher levels of apoptotic cell death when ErPC3 was combined with radiotherapy.
Conclusions: Our data suggest that constitutive Akt activation and survival are controlled by different different molecular mechanisms in the two prostate cancer cell lines - one which is sensitive to the Akt-inhibitor ErPC3 and one which is more sensitive to the PI3K-inhibitor LY294002. Our findings underline the importance for the definition of predictive biomarkers that allow the selection patients that may benefit from the treatment with a specific signal transduction modifier.
Figures


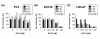
Similar articles
-
Long-term androgen-ablation causes increased resistance to PI3K/Akt pathway inhibition in prostate cancer cells.Prostate. 2004 Feb 15;58(3):259-68. doi: 10.1002/pros.10332. Prostate. 2004. PMID: 14743465
-
The translocator protein radioligand 18F-DPA-714 monitors antitumor effect of erufosine in a rat 9L intracranial glioma model.J Nucl Med. 2013 Dec;54(12):2125-31. doi: 10.2967/jnumed.112.118794. Epub 2013 Nov 8. J Nucl Med. 2013. PMID: 24212976
-
ABT-737 and erufosine combination against castration-resistant prostate cancer: a promising but cell-type specific response associated with the modulation of anti-apoptotic signaling.Anticancer Drugs. 2019 Apr;30(4):383-393. doi: 10.1097/CAD.0000000000000736. Anticancer Drugs. 2019. PMID: 30557204
-
Anticancer mechanisms and clinical application of alkylphospholipids.Biochim Biophys Acta. 2013 Mar;1831(3):663-74. doi: 10.1016/j.bbalip.2012.10.008. Epub 2012 Nov 5. Biochim Biophys Acta. 2013. PMID: 23137567 Review.
-
RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells.Ann N Y Acad Sci. 2010 Jul;1201:129-36. doi: 10.1111/j.1749-6632.2010.05613.x. Ann N Y Acad Sci. 2010. PMID: 20649549 Free PMC article. Review.
Cited by
-
Harnessing the potential of multimodal radiotherapy in prostate cancer.Nat Rev Urol. 2020 Jun;17(6):321-338. doi: 10.1038/s41585-020-0310-3. Epub 2020 May 1. Nat Rev Urol. 2020. PMID: 32358562 Review.
-
Combination treatment with naftopidil increases the efficacy of radiotherapy in PC-3 human prostate cancer cells.J Cancer Res Clin Oncol. 2017 Jun;143(6):933-939. doi: 10.1007/s00432-017-2367-9. Epub 2017 Feb 27. J Cancer Res Clin Oncol. 2017. PMID: 28243746 Free PMC article.
-
Microenvironment and radiation therapy.Biomed Res Int. 2013;2013:685308. doi: 10.1155/2013/685308. Epub 2012 Dec 4. Biomed Res Int. 2013. PMID: 23509762 Free PMC article. Review.
-
In vitro effect of molluscan hemocyanins on CAL-29 and T-24 bladder cancer cell lines.Biomed Rep. 2013 Mar;1(2):235-238. doi: 10.3892/br.2012.46. Epub 2012 Dec 10. Biomed Rep. 2013. PMID: 24648926 Free PMC article.
-
Simultaneous perturbation of the MAPK and the PI3K/mTOR pathways does not lead to increased radiosensitization.Radiat Oncol. 2015 Oct 24;10:214. doi: 10.1186/s13014-015-0514-5. Radiat Oncol. 2015. PMID: 26498922 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

