"pp65 antigenemia and real time polymerase chain reaction (PCR) based-study to determine the prevalence of human cytomegalovirus (HCMV) in kidney donors and recipients with follow-up studies"
- PMID: 21080922
- PMCID: PMC2998481
- DOI: 10.1186/1743-422X-7-322
"pp65 antigenemia and real time polymerase chain reaction (PCR) based-study to determine the prevalence of human cytomegalovirus (HCMV) in kidney donors and recipients with follow-up studies"
Abstract
Background: The present study was undertaken to determine the rate of occurrence of Human cytomegalovirus (HCMV) among kidney transplant recipients and donors by application of direct detection methods and to understand HCMV infection/disease development among transplanted patients as a prospective study.
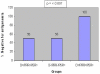
Results: Peripheral blood samples collected from 76 kidney donors and 76 recipients from September 2007 to August 2009 were subjected to pp65 antigenemia and Quantitative real-time PCR (qRT-PCR) assays. Data were analyzed under Group A, B and C. Group A was further divided into sub-groups I, II, III, IV, and V for better understanding. Three, one and two donors in sub-group I, III, IV of Group A tested positive for real time PCR respectively. One recipient from group III tested positive for HCMV by qRT- PCR prior transplantation and remained positive one month post-transplantation. Three other recipients, tested negative prior to transplantation became positive a month after transplantation. Group B consisted of 18 donor-recipient pairs and one of the donor tested positive for HCMV by qRT-PCR. Eight recipients tested positive for HCMV one month after transplantation. The pp65 positivity and HCMV DNA load was high among group C recipients who mostly had symptoms of active disease. Significantly high values of pp65 antigenemia were observed among recipients of sub-group II (non-parametric chi-square test p = 0.007). Positive correlation between pp65 antigenemia and qRT-PCR value was observed. Thirty three of the recipients with disease treated with Valgancyclovir showed improved clinical outcome.
Conclusion: Our study showed that a significant proportion of kidney recipients develop HCMV infection following renal transplantation in spite of the absence of HCMV among donors. pp65 antigenemia assay and qRT- PCR methods can be applied to detect HCMV among kidney donors and recipients to monitor development of disease and these assays were predicative of HCMV infection among them. Clinical resistant to valganciclovir was not observed.
Figures



References
-
- Costa SC, Miranda SR, Alves G, Rossi CL, Fiqueiredo LT, Costa FF. Donated organs as a source of cytomegalovirus (CMV) in renal transplant patients. Braz J Med Biol Res. 1994;27:2573–8. - PubMed
-
- Cliona M, Helen E. Immunotherapy for malignancies and viral infections. Current Opinion in Organ. Curr Opin Organ Transplant. 2000;5:197–202. doi: 10.1097/00075200-200009000-00005. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

