Germline transformation of the stalk-eyed fly, Teleopsis dalmanni
- PMID: 21080934
- PMCID: PMC2999598
- DOI: 10.1186/1471-2199-11-86
Germline transformation of the stalk-eyed fly, Teleopsis dalmanni
Abstract
Background: Stalk-eyed flies of the family Diopsidae have proven to be an excellent model organism for studying the evolution of ornamental sexual traits. In diopsid flies the eyes and antennae are borne at the end of lateral head projections called 'eye-stalks'. Eyespan, the distance between the eyes, and the degree of sexual dimorphism in eyespan vary considerably between species and several sexually dimorphic species show sexual selection through female mate preference for males with exaggerated eyespan. Relatively little is known about the molecular genetic basis of intra- or inter-species variation in eyespan, eye-stalk development or growth regulation in diopsids. Molecular approaches including comparative developmental analyses, EST screening and QTL mapping have identified potential candidate loci for eyespan regulation in the model species Teleopsis dalmanni. Functional analyses of these genes to confirm and fully characterise their roles in eye-stalk growth require the development of techniques such as germline transformation to manipulate gene activity in vivo.
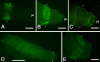
Results: We used in vivo excision assays to identify transposon vector systems with the activity required to mediate transgenesis in T. dalmanni. Mariner based vectors showed no detectable excision while both Minos and piggyBac were active in stalk-eyed fly embryos. Germline transformation with an overall efficiency of 4% was achieved using a Minos based vector and the 3xP3-EGFP marker construct. Chromosomal insertion of constructs was confirmed by Southern blot analysis. Both autosomal and X-linked inserts were recovered. A homozygous stock, established from one of the X-linked inserts, has maintained stable expression for eight generations.
Conclusions: We have performed stable germline transformation of a stalk-eyed fly, T. dalmanni. This is the first transgenic protocol to be developed in an insect species that exhibits an exaggerated male sexual trait. Transgenesis will enable the development of a range of techniques for analysing gene function in this species and so provide insight into the mechanisms underlying the development of a morphological trait subject to sexual selection. Our X-linked insertion line will permit the sex of live larvae to be determined. This will greatly facilitate the identification of genes which are differentially expressed during eye-stalk development in males and females.
Figures


References
-
- Darwin C. The Descent of Man, and Selection in Relation to Sex. London: Murray; 1871.
-
- Andersson M. Sexual Selection. New Jersey: Princeton University Press; 1994.
-
- Wilkinson GS. In: Model Systems in Behavioural Ecology. Integrating Conceptual, Theoretical, and Empirical Approaches. Dugatkin LA, editor. Princeton: Princeton University Press; 2001. Genetic consequences of sexual selection in stalk-eyed flies; pp. 72–91.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

