Chromatin structure as a mediator of aging
- PMID: 21081125
- PMCID: PMC3988783
- DOI: 10.1016/j.febslet.2010.11.016
Chromatin structure as a mediator of aging
Abstract
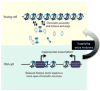
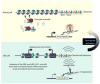
The aging process is characterized by gradual changes to an organism's macromolecules, which negatively impacts biological processes. The complex macromolecular structure of chromatin regulates all nuclear processes requiring access to the DNA sequence. As such, maintenance of chromatin structure is an integral component to deter premature aging. In this review, we describe current research that links aging to chromatin structure. Histone modifications influence chromatin compaction and gene expression and undergo many changes during aging. Histone protein levels also decline during aging, dramatically affecting chromatin structure. Excitingly, lifespan can be extended by manipulations that reverse the age-dependent changes to chromatin structure, indicating the pivotal role chromatin structure plays during aging.
Copyright © 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–60. - PubMed
-
- Villeponteau B. The heterochromatin loss model of aging. Exp Gerontol. 1997;32:383–94. - PubMed
-
- Oberdoerffer P, Sinclair DA. The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol. 2007;8:692–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

