The interactome of a PTB domain-containing adapter protein, Odin, revealed by SILAC
- PMID: 21081186
- PMCID: PMC3205450
- DOI: 10.1016/j.jprot.2010.11.006
The interactome of a PTB domain-containing adapter protein, Odin, revealed by SILAC
Abstract
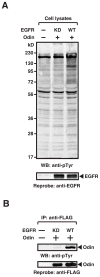
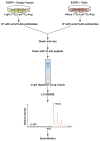
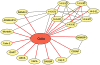
Signal transduction pathways are tightly controlled by positive and negative regulators. We have previously identified Odin (also known as ankyrin repeat and sterile alpha motif domain-containing 1A; gene symbol ANKS1A) as a negative regulator of growth factor signaling; however, the mechanisms through which Odin regulates these pathways remain to be elucidated. To determine how Odin negatively regulates growth factor signaling, we undertook a proteomic approach to systematically identify proteins that interact with Odin using the SILAC strategy. In this study, we identified 18 molecules that were specifically associated in a protein complex with Odin. Our study established that the complete family of 14-3-3 proteins occur in a protein complex with Odin, which is also supported by earlier reports that identified a few members of the 14-3-3 family as Odin interactors. Among the novel protein interactors of Odin were CD2-associated protein, SH3 domain kinase binding protein 1 and DAB2 interacting protein. We confirmed 8 of the eighteen interactions identified in the Odin protein complex by co-immunoprecipitation experiments. Finally, a literature-based network analysis revealed that Odin interacting partners are involved in various cellular processes, some of which are key molecules in regulating receptor endocytosis.
Copyright © 2010 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures


References
-
- Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I. Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature. 2002;416:183–7. - PubMed
-
- Szymkiewicz I, Kowanetz K, Soubeyran P, Dinarina A, Lipkowitz S, Dikic I. CIN85 participates in Cbl-b-mediated down-regulation of receptor tyrosine kinases. J Biol Chem. 2002;277:39666–72. - PubMed
-
- Petrelli A, Gilestro GF, Lanzardo S, Comoglio PM, Migone N, Giordano S. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature. 2002;416:187–90. - PubMed
-
- Steen H, Kuster B, Fernandez M, Pandey A, Mann M. Tyrosine phosphorylation mapping of the epidermal growth factor receptor signaling pathway. J Biol Chem. 2002;277:1031–9. - PubMed
-
- Pandey A, Blagoev B, Kratchmarova I, Fernandez M, Nielsen M, Kristiansen TZ, et al. Cloning of a novel phosphotyrosine binding domain containing molecule, Odin, involved in signaling by receptor tyrosine kinases. Oncogene. 2002;21:8029–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

