Cardiovascular effects of erythropoietin an update
- PMID: 21081221
- PMCID: PMC3907121
- DOI: 10.1016/B978-0-12-385061-4.00009-X
Cardiovascular effects of erythropoietin an update
Abstract
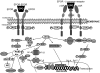
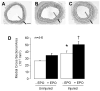
Erythropoietin (EPO) is a therapeutic product of recombinant DNA technology and it has been in clinical use as stimulator of erythropoiesis over the last two decades. Identification of EPO and its receptor (EPOR) in the cardiovascular system expanded understanding of physiological and pathophysiological role of EPO. In experimental models of cardiovascular and cerebrovascular disorders, EPO exerts protection either by preventing apoptosis of cardiac myocytes, smooth muscle cells, and endothelial cells, or by increasing endothelial production of nitric oxide. In addition, EPO stimulates mobilization of progenitor cells from bone marrow thereby accelerating repair of injured endothelium and neovascularization. A novel signal transduction pathway involving EPOR--β-common heteroreceptor is postulated to enhance EPO-mediated tissue protection. A better understanding of the role of β-common receptor signaling as well as development of novel analogs of EPO with enhanced nonhematopoietic protective effects may expand clinical application of EPO in prevention and treatment of cardiovascular and cerebrovascular disorders.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Current perspectives on protective roles of erythropoietin in cardiovascular system: erythropoietin receptor as a novel therapeutic target.Tohoku J Exp Med. 2012 Jun;227(2):83-91. doi: 10.1620/tjem.227.83. Tohoku J Exp Med. 2012. PMID: 22688525 Review.
-
Protective effects of erythropoietin in cardiac ischemia: from bench to bedside.J Am Coll Cardiol. 2006 Dec 5;48(11):2161-7. doi: 10.1016/j.jacc.2006.08.031. Epub 2006 Nov 9. J Am Coll Cardiol. 2006. PMID: 17161240 Review.
-
Emergence of the erythropoietin/erythropoietin receptor system as a novel cardiovascular therapeutic target.J Cardiovasc Pharmacol. 2011 Dec;58(6):570-4. doi: 10.1097/FJC.0b013e318235e7bb. J Cardiovasc Pharmacol. 2011. PMID: 21934628
-
Erythropoietin and treatment of non-anemic conditions--cardiovascular protection.Semin Hematol. 2007 Jul;44(3):212-7. doi: 10.1053/j.seminhematol.2007.04.008. Semin Hematol. 2007. PMID: 17631185 Review.
-
Erythropoietin improves histological and functional outcomes after traumatic brain injury in mice in the absence of the neural erythropoietin receptor.J Neurotrauma. 2010 Jan;27(1):205-15. doi: 10.1089/neu.2009.1001. J Neurotrauma. 2010. PMID: 19715391 Free PMC article.
Cited by
-
Regulation of TLR4 expression mediates the attenuating effect of erythropoietin on inflammation and myocardial fibrosis in rat heart.Int J Mol Med. 2018 Sep;42(3):1436-1444. doi: 10.3892/ijmm.2018.3707. Epub 2018 May 25. Int J Mol Med. 2018. PMID: 29845292 Free PMC article.
-
Suppression of Inflammatory Cardiac Cytokine Network in Rats with Untreated Obesity and Pre-Diabetes by AT2 Receptor Agonist NP-6A4.Front Pharmacol. 2021 Jun 18;12:693167. doi: 10.3389/fphar.2021.693167. eCollection 2021. Front Pharmacol. 2021. PMID: 34220518 Free PMC article.
-
Association of Serum Erythropoietin With Cardiovascular Events, Kidney Function Decline, and Mortality: The Health Aging and Body Composition Study.Circ Heart Fail. 2016 Jan;9(1):e002124. doi: 10.1161/CIRCHEARTFAILURE.115.002124. Circ Heart Fail. 2016. PMID: 26721912 Free PMC article.
-
Heart Muscle Microphysiological System for Cardiac Liability Prediction of Repurposed COVID-19 Therapeutics.Front Pharmacol. 2021 Aug 4;12:684252. doi: 10.3389/fphar.2021.684252. eCollection 2021. Front Pharmacol. 2021. PMID: 34421592 Free PMC article.
-
p42/p44-MAPK and PI3K are sufficient for IL-6 family cytokines/gp130 to signal to hypertrophy and survival in cardiomyocytes in the absence of JAK/STAT activation.Cell Signal. 2013 Apr;25(4):898-909. doi: 10.1016/j.cellsig.2012.12.008. Epub 2012 Dec 23. Cell Signal. 2013. PMID: 23268184 Free PMC article.
References
-
- Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. - PubMed
-
- Akimoto T, Kusano E, Ito C, Yanagiba S, Inoue M, Amemiya M, Ando Y, Asano Y. Involvement of erythropoietin-induced cytosolic free calcium mobilization in activation of mitogen-activated protein kinase and DNA synthesis in vascular smooth muscle cells. J Hypertens. 2001;19:193–202. - PubMed
-
- Akimoto T, Kusano E, Muto S, Fujita N, Okada K, Saito T, Komatsu N, Ono S, Ebata S, Ando Y, Homma S, Asano Y. The effect of erythropoietin on interleukin-1beta mediated increase in nitric oxide synthesis in vascular smooth muscle cells. J Hypertens. 1999;17:1249–1256. - PubMed
-
- Ammarguellat F, Gogusev J, Drueke TB. Direct effect of erythropoietin on rat vascular smooth-muscle cell via a putative erythropoietin receptor. Nephrol Dial Transplant. 1996;11:687–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

