Tamm-Horsfall glycoprotein interacts with renal outer medullary potassium channel ROMK2 and regulates its function
- PMID: 21081491
- PMCID: PMC3023518
- DOI: 10.1074/jbc.M110.149880
Tamm-Horsfall glycoprotein interacts with renal outer medullary potassium channel ROMK2 and regulates its function
Abstract
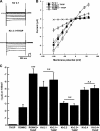
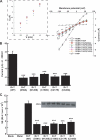
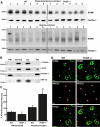
Tamm-Horsfall glycoprotein (THGP) or Uromodulin is a membrane protein exclusively expressed along the thick ascending limb (TAL) and early distal convoluted tubule (DCT) of the nephron. Mutations in the THGP encoding gene result in Familial Juvenile Hyperuricemic Nephropathy (FJHN), Medullary Cystic Kidney Disease type 2 (MCKD-2), and Glomerulocystic Kidney Disease (GCKD). The physicochemical and biological properties of THGP have been studied extensively, but its physiological function in the TAL remains obscure. We performed yeast two-hybrid screening employing a human kidney cDNA library and identified THGP as a potential interaction partner of the renal outer medullary potassium channel (ROMK2), a key player in the process of salt reabsorption along the TAL. Functional analysis by electrophysiological techniques in Xenopus oocytes showed a strong increase in ROMK current amplitudes when co-expressed with THGP. The effect of THGP was specific for ROMK2 and did not influence current amplitudes upon co-expression with Kir2.x, inward rectifier potassium channels related to ROMK. Single channel conductance and open probability of ROMK2 were not altered by co-expression of THGP, which instead increased surface expression of ROMK2 as determined by patch clamp analysis and luminometric surface quantification, respectively. Despite preserved interaction with ROMK2, disease-causing THGP mutants failed to increase its current amplitude and surface expression. THGP(-/-) mice exhibited increased ROMK accumulation in intracellular vesicular compartments when compared with WT animals. Therefore, THGP modulation of ROMK function confers a new role of THGP on renal ion transport and may contribute to salt wasting observed in FJHN/MCKD-2/GCKD patients.
Figures



Similar articles
-
Novel UMOD mutations in familial juvenile hyperuricemic nephropathy lead to abnormal uromodulin intracellular trafficking.Gene. 2013 Dec 1;531(2):363-9. doi: 10.1016/j.gene.2013.08.041. Epub 2013 Aug 27. Gene. 2013. PMID: 23988501
-
Allelism of MCKD, FJHN and GCKD caused by impairment of uromodulin export dynamics.Hum Mol Genet. 2003 Dec 15;12(24):3369-84. doi: 10.1093/hmg/ddg353. Epub 2003 Oct 21. Hum Mol Genet. 2003. PMID: 14570709
-
The glycolytic enzymes glyceraldehyde 3-phosphate dehydrogenase and enolase interact with the renal epithelial K+ channel ROMK2 and regulate its function.Cell Physiol Biochem. 2011;28(4):663-72. doi: 10.1159/000335761. Epub 2011 Dec 14. Cell Physiol Biochem. 2011. PMID: 22178878
-
Renal outer medullary potassium channel knockout models reveal thick ascending limb function and dysfunction.Clin Exp Nephrol. 2012 Feb;16(1):49-54. doi: 10.1007/s10157-011-0495-0. Epub 2011 Nov 1. Clin Exp Nephrol. 2012. PMID: 22038261 Review.
-
Uromodulin: old friend with new roles in health and disease.Pediatr Nephrol. 2014 Jul;29(7):1151-8. doi: 10.1007/s00467-013-2563-z. Epub 2013 Jul 24. Pediatr Nephrol. 2014. PMID: 23880785 Review.
Cited by
-
Next generation sequencing search for uromodulin gene variants related with impaired renal function.Mol Biol Rep. 2015 Sep;42(9):1353-8. doi: 10.1007/s11033-015-3883-9. Epub 2015 Jun 4. Mol Biol Rep. 2015. PMID: 26040415
-
Nephrolithiasis secondary to inherited defects in the thick ascending loop of henle and connecting tubules.Urolithiasis. 2019 Feb;47(1):43-56. doi: 10.1007/s00240-018-1097-z. Epub 2018 Nov 20. Urolithiasis. 2019. PMID: 30460527 Review.
-
Common noncoding UMOD gene variants induce salt-sensitive hypertension and kidney damage by increasing uromodulin expression.Nat Med. 2013 Dec;19(12):1655-60. doi: 10.1038/nm.3384. Epub 2013 Nov 3. Nat Med. 2013. PMID: 24185693 Free PMC article.
-
Role of Uromodulin in Salt-Sensitive Hypertension.Hypertension. 2022 Nov;79(11):2419-2429. doi: 10.1161/HYPERTENSIONAHA.122.19888. Epub 2022 Sep 12. Hypertension. 2022. PMID: 36378920 Free PMC article. Review.
-
Autosomal dominant tubulointerstitial kidney disease: A review.Am J Med Genet C Semin Med Genet. 2022 Sep;190(3):309-324. doi: 10.1002/ajmg.c.32008. Epub 2022 Oct 17. Am J Med Genet C Semin Med Genet. 2022. PMID: 36250282 Free PMC article. Review.
References
-
- Malagolini N., Cavallone D., Serafini-Cessi F. (1997) Kidney Int. 52, 1340–1350 - PubMed
-
- Devuyst O., Dahan K., Pirson Y. (2005) Nephrol. Dial Transplant. 20, 1290–1294 - PubMed
-
- Rindler M. J., Naik S. S., Li N., Hoops T. C., Peraldi M. N. (1990) J. Biol. Chem. 265, 20784–20789 - PubMed
-
- Cavallone D., Malagolini N., Serafini-Cessi F. (2001) Biochem. Biophys. Res. Commun. 280, 110–114 - PubMed
-
- Orskov I., Ferencz A., Orskov F. (1980) Lancet 1, 887 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

