Contribution of CmeG to antibiotic and oxidative stress resistance in Campylobacter jejuni
- PMID: 21081547
- PMCID: PMC3001851
- DOI: 10.1093/jac/dkq418
Contribution of CmeG to antibiotic and oxidative stress resistance in Campylobacter jejuni
Abstract
Objectives: campylobacter jejuni is a leading foodborne pathogen worldwide and its resistance to antimicrobials is a major concern for public health. The cmeG (Cj1375) gene in C. jejuni encodes a putative efflux transporter of the major facilitator family, but its function in antimicrobial resistance has not been determined. This study aimed to characterize the function of CmeG in conferring resistance to antibiotics and oxidative stress.
Methods: the cmeG gene (Cj1375) in C. jejuni was inactivated by insertional mutagenesis and overexpressed by cloning with a shuttle vector. These constructs were compared with the wild-type strain using antimicrobial susceptibility tests and drug accumulation assays.
Results: the cmeG mutation reduced bacterial growth and rendered C. jejuni more susceptible to ciprofloxacin, erythromycin, gentamicin, tetracycline, rifampicin, ethidium bromide and cholic acid as well as hydrogen peroxide, and in trans complementation restored the susceptibility to near wild-type level. RT-PCR showed that cmeG is co-transcribed with its downstream gene cmeH (Cj1376) encoding a putative periplasmic protein, but mutation of cmeH alone did not affect the susceptibility to antibiotics. Notably, overexpression of the cmeGH operon in C. jejuni NCTC 11168 significantly increased its resistance to fluoroquinolones. In addition, the cmeG mutant accumulated more EtBr and ciprofloxacin than the wild-type strain.
Conclusions: these results indicate that CmeG functions as a multidrug efflux transporter contributing to antibiotic resistance and oxidative defence in Campylobacter.
Figures
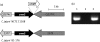

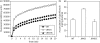
References
-
- Friedman CR, Neimann J, Wegener HC, et al. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser MJ, editors. Campylobacter. Washington, DC, USA: ASM Press; 2000. pp. 121–38.
-
- Allos BM. Campylobacter jejuni infections: update on emerging issues and trends. Clin Infect Dis. 2001;32:1201–6. - PubMed
-
- Poole K. Efflux-mediated antimicrobial resistance. J Antimicrob Chemother. 2005;56:20–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

