Impaired consciousness in temporal lobe seizures: role of cortical slow activity
- PMID: 21081551
- PMCID: PMC2995886
- DOI: 10.1093/brain/awq316
Impaired consciousness in temporal lobe seizures: role of cortical slow activity
Abstract
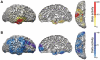
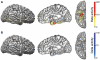
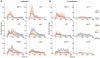
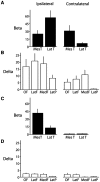
Impaired consciousness requires altered cortical function. This can occur either directly from disorders that impair widespread bilateral regions of the cortex or indirectly through effects on subcortical arousal systems. It has therefore long been puzzling why focal temporal lobe seizures so often impair consciousness. Early work suggested that altered consciousness may occur with bilateral or dominant temporal lobe seizure involvement. However, other bilateral temporal lobe disorders do not impair consciousness. More recent work supports a 'network inhibition hypothesis' in which temporal lobe seizures disrupt brainstem-diencephalic arousal systems, leading indirectly to depressed cortical function and impaired consciousness. Indeed, prior studies show subcortical involvement in temporal lobe seizures and bilateral frontoparietal slow wave activity on intracranial electroencephalography. However, the relationships between frontoparietal slow waves and impaired consciousness and between cortical slowing and fast seizure activity have not been directly investigated. We analysed intracranial electroencephalography recordings during 63 partial seizures in 26 patients with surgically confirmed mesial temporal lobe epilepsy. Behavioural responsiveness was determined based on blinded review of video during seizures and classified as impaired (complex-partial seizures) or unimpaired (simple-partial seizures). We observed significantly increased delta-range 1-2 Hz slow wave activity in the bilateral frontal and parietal neocortices during complex-partial compared with simple-partial seizures. In addition, we confirmed prior work suggesting that propagation of unilateral mesial temporal fast seizure activity to the bilateral temporal lobes was significantly greater in complex-partial than in simple-partial seizures. Interestingly, we found that the signal power of frontoparietal slow wave activity was significantly correlated with the temporal lobe fast seizure activity in each hemisphere. Finally, we observed that complex-partial seizures were somewhat more common with onset in the language-dominant temporal lobe. These findings provide direct evidence for cortical dysfunction in the form of bilateral frontoparietal slow waves associated with impaired consciousness in temporal lobe seizures. We hypothesize that bilateral temporal lobe seizures may exert a powerful inhibitory effect on subcortical arousal systems. Further investigations will be needed to fully determine the role of cortical-subcortical networks in ictal neocortical dysfunction and may reveal treatments to prevent this important negative consequence of temporal lobe epilepsy.
Figures



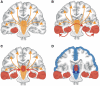
References
-
- Arthuis M, Valton L, Regis J, Chauvel P, Wendling F, Naccache L, et al. Impaired consciousness during temporal lobe seizures is related to increased long-distance cortical-subcortical synchronization. Brain. 2009;132:2091–101. - PubMed
-
- Bancaud J, Brunet-Bourgin F, Chauvel P, Halgren E. Anatomical origin of deja vu and vivid 'memories' in human temporal lobe epilepsy. Brain. 1994;117(Pt 1):71–90. - PubMed
-
- Bertashius KM. Propagation of human complex-partial seizures: a correlation analysis. Electroencephalogr Clin Neurophysiol. 1991;78:333–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

