Progesterone receptor membrane component 1 inhibits the activity of drug-metabolizing cytochromes P450 and binds to cytochrome P450 reductase
- PMID: 21081644
- PMCID: PMC3061357
- DOI: 10.1124/mol.110.068478
Progesterone receptor membrane component 1 inhibits the activity of drug-metabolizing cytochromes P450 and binds to cytochrome P450 reductase
Abstract
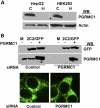
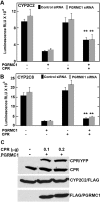
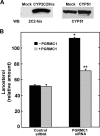
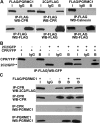
Progesterone receptor membrane component 1 (PGRMC1) has been shown to interact with several cytochromes P450 (P450s) and to activate enzymatic activity of P450s involved in sterol biosynthesis. We analyzed the interactions of PGRMC1 with the drug-metabolizing P450s, CYP2C2, CYP2C8, and CYP3A4, in transfected cells. Based on coimmunoprecipitation assays, PGRMC1 bound efficiently to all three P450s, and binding to the catalytic cytoplasmic domain of CYP2C2 was much more efficient than to a chimera containing only the N-terminal transmembrane domain. Down-regulation of PGRMC1 expression levels in human embryonic kidney 293 and HepG2 cell lines stably expressing PGRMC1-specific small interfering RNA had no effect on the endoplasmic reticulum localization and expression levels of P450s, whereas enzymatic activities of CYP2C2, CYP2C8, and CYP3A4 were slightly higher in PGRMC1-deficient cells. Cotransfection of cells with P450s and PGRMC1 resulted in PGRMC1 concentration-dependent inhibition of the P450 activities, and this inhibition was partially reversed by increased expression of the P450 reductase (CPR). In contrast, CYP51 activity was decreased by down-regulation of PGRMC1 and expression of PGRMC1 in the PGRMC1-deficient cells increased CYP51 activity. In cells cotransfected with CPR and PGRMC1, strong binding of CPR to PGRMC1 was observed; however, in the presence of CYP2C2, interaction of PGRMC1 with CPR was significantly reduced, suggesting that CYP2C2 competes with CPR for binding to PGRMC1. These data show that in contrast to sterol synthesizing P450, PGRMC1 is not required for the activities of several drug-metabolizing P450s, and its overexpression inhibits those P450 activities. Furthermore, PGRMC1 binds to CPR, which may influence P450 activity.
Figures





References
-
- Ahmed IS, Rohe HJ, Twist KE, Mattingly MN, Craven RJ. (2010) Progesterone receptor membrane component 1 (Pgrmc1): a heme-1 domain protein that promotes tumorigenesis and is inhibited by a small molecule. J Pharmacol Exp Ther 333:564–573 - PubMed
-
- Ahn K, Szczesna-Skorupa E, Kemper B. (1993) The amino-terminal 29 amino acids of cytochrome P450 2C1 are sufficient for retention in the endoplasmic reticulum. J Biol Chem 268:18726–18733 - PubMed
-
- Backes WL, Kelley RW. (2003) Organization of multiple cytochrome P450s with NADPH-cytochrome P450 reductase in membranes. Pharmacol Ther 98:221–233 - PubMed
-
- Bridges A, Gruenke L, Chang YT, Vakser IA, Loew G, Waskell L. (1998) Identification of the binding site on cytochrome P450 2B4 for cytochrome b5 and cytochrome P450 reductase. J Biol Chem 273:17036–17049 - PubMed
-
- Cahill MA. (2007) Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol 105:16–36 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

