A pharmacodynamic study of docetaxel in combination with the P-glycoprotein antagonist tariquidar (XR9576) in patients with lung, ovarian, and cervical cancer
- PMID: 21081657
- PMCID: PMC3071989
- DOI: 10.1158/1078-0432.CCR-10-1725
A pharmacodynamic study of docetaxel in combination with the P-glycoprotein antagonist tariquidar (XR9576) in patients with lung, ovarian, and cervical cancer
Abstract
Purpose: P-glycoprotein (Pgp) antagonists have been difficult to develop because of complex pharmacokinetic interactions and a failure to show meaningful results. Here we report the results of a pharmacokinetic and pharmacodynamic trial using a third-generation, potent, noncompetitive inhibitor of Pgp, tariquidar (XR9576), in combination with docetaxel.
Experimental design: In the first treatment cycle, the pharmacokinetics of docetaxel (40 mg/m(2)) were evaluated after day 1 and day 8 doses, which were administered with or without tariquidar (150 mg). (99m)Tc-sestamibi scanning and CD56(+) mononuclear cell rhodamine efflux assays were conducted to assess Pgp inhibition. In subsequent cycles, 75 mg/m(2) docetaxel was administered with 150 mg tariquidar every 3 weeks.
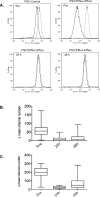
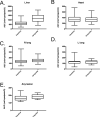
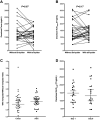
Results: Forty-eight patients were enrolled onto the trial. Nonhematologic grade 3/4 toxicities in 235 cycles were minimal. Tariquidar inhibited Pgp-mediated rhodamine efflux from CD56(+) cells and reduced (99m)Tc-sestamibi clearance from the liver. There was striking variability in basal sestamibi uptake; a 12% to 24% increase in visible lesions was noted in 8 of 10 patients with lung cancer. No significant difference in docetaxel disposition was observed in pairwise comparison with and without tariquidar. Four partial responses (PR) were seen (4/48); 3 in the non-small cell lung cancer (NSCLC) cohort, measuring 40%, 57%, and 67% by RECIST, and 1 PR in a patient with ovarian cancer.
Conclusions: Tariquidar is well tolerated, with less observed systemic pharmacokinetic interaction than previous Pgp antagonists. Variable effects of tariquidar on retention of sestamibi in imageable lung cancers suggest that follow-up studies assessing tumor drug uptake in this patient population would be worthwhile.
©2010 AACR.
Figures

Similar articles
-
A phase I study of the P-glycoprotein antagonist tariquidar in combination with vinorelbine.Clin Cancer Res. 2009 May 15;15(10):3574-82. doi: 10.1158/1078-0432.CCR-08-0938. Epub 2009 May 5. Clin Cancer Res. 2009. PMID: 19417029 Free PMC article. Clinical Trial.
-
Pharmacokinetic and pharmacodynamic study of tariquidar (XR9576), a P-glycoprotein inhibitor, in combination with doxorubicin, vinorelbine, or docetaxel in children and adolescents with refractory solid tumors.Cancer Chemother Pharmacol. 2015 Dec;76(6):1273-83. doi: 10.1007/s00280-015-2845-1. Epub 2015 Oct 20. Cancer Chemother Pharmacol. 2015. PMID: 26486517 Free PMC article. Clinical Trial.
-
A pharmacodynamic study of the P-glycoprotein antagonist CBT-1® in combination with paclitaxel in solid tumors.Oncologist. 2012;17(4):512. doi: 10.1634/theoncologist.2012-0080. Epub 2012 Mar 13. Oncologist. 2012. PMID: 22416063 Free PMC article. Clinical Trial.
-
Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor.Expert Rev Anticancer Ther. 2007 Apr;7(4):447-59. doi: 10.1586/14737140.7.4.447. Expert Rev Anticancer Ther. 2007. PMID: 17428165 Review.
-
Phase II study of first-line sequential chemotherapy with gemcitabine-carboplatin followed by docetaxel in patients with advanced non-small cell lung cancer.Oncology. 2005;68(4-6):382-90. doi: 10.1159/000086979. Epub 2005 Jul 12. Oncology. 2005. PMID: 16020967 Review.
Cited by
-
Disrupting P-glycoprotein function in clinical settings: what can we learn from the fundamental aspects of this transporter?Am J Cancer Res. 2016 Aug 1;6(8):1583-98. eCollection 2016. Am J Cancer Res. 2016. PMID: 27648351 Free PMC article. Review.
-
Comprehensive Evaluation of Multiple Approaches Targeting ABCB1 to Resensitize Docetaxel-Resistant Prostate Cancer Cell Lines.Int J Mol Sci. 2022 Dec 30;24(1):666. doi: 10.3390/ijms24010666. Int J Mol Sci. 2022. PMID: 36614114 Free PMC article.
-
Lapatinib and poziotinib overcome ABCB1-mediated paclitaxel resistance in ovarian cancer.PLoS One. 2021 Aug 4;16(8):e0254205. doi: 10.1371/journal.pone.0254205. eCollection 2021. PLoS One. 2021. PMID: 34347777 Free PMC article.
-
Targeting P-glycoprotein: Investigation of piperine analogs for overcoming drug resistance in cancer.Sci Rep. 2017 Aug 11;7(1):7972. doi: 10.1038/s41598-017-08062-2. Sci Rep. 2017. PMID: 28801675 Free PMC article.
-
Revisiting the role of ABC transporters in multidrug-resistant cancer.Nat Rev Cancer. 2018 Jul;18(7):452-464. doi: 10.1038/s41568-018-0005-8. Nat Rev Cancer. 2018. PMID: 29643473 Free PMC article. Review.
References
-
- Fojo A, Akiyama S, Gottesman MM, Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985;45:3002–7. - PubMed
-
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. - PubMed
-
- Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8:411–24. - PubMed
-
- Rowinsky EK, Smith L, Wang YM, et al. Phase I and pharmacokinetic study of paclitaxel in combination with biricodar, a novel agent that reverses multidrug resistance conferred by overexpression of both MDR1 and MRP. J Clin Oncol. 1998;16:2964–76. - PubMed
-
- Hyafil F, Vergely C, Du Vignaud P, Grand-Perret T. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993;53:4595–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

