The lipoxygenase-dependent oxygenation of lipid body membranes is promoted by a patatin-type phospholipase in cucumber cotyledons
- PMID: 21081663
- PMCID: PMC3003817
- DOI: 10.1093/jxb/erq310
The lipoxygenase-dependent oxygenation of lipid body membranes is promoted by a patatin-type phospholipase in cucumber cotyledons
Abstract
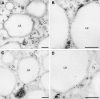
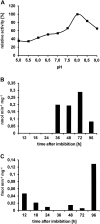
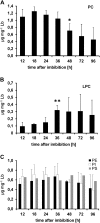
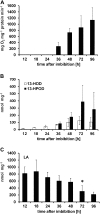
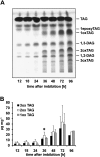
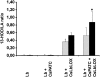
Oilseed germination is characterized by the mobilization of storage lipids as a carbon and energy source for embryonic growth. In addition to storage lipid degradation in germinating oilseeds via the direct action of a triacylglycerol lipase (TGL) on the storage lipids, a second degradation pathway that is dependent on a specific lipid body trilinoleate 13-lipoxygenase (13-LOX) has been proposed in several plant species. The activity of this specific 13-LOX leads first to the formation of ester lipid hydroperoxides. These hydroperoxy fatty acids are then preferentially cleaved off by a TGL and serve as a substrate for glyoxysomal β-oxidation. As a prerequisite for triacylglycerol (TAG) mobilization, a partial degradation of the phospholipid monolayer and/or membrane proteins of the oil body has been discussed. Evidence has now been found for both processes: partial degradation of the proteins caleosin and oleosin was observed and simultaneously a patatin-like protein together with transient phospholipase (PLase) activity could be detected at the oil body membranes during germination. Moreover, in vitro experiments with isolated oil bodies from mature seeds revealed that the formation of 13-LOX-derived lipid peroxides in lipid body membranes is increased after incubation with the purified recombinant patatin-like protein. These experiments suggest that in vivo the degradation of storage lipids in cucumber cotyledons is promoted by the activity of a specific oil body PLase, which leads to an increased decomposition of the oil body membrane by the 13-LOX and thereby TAGs may be better accessible to LOX and TGL.
Figures

References
-
- Balkenhohl T, Kühn H, Wasternack C, Feussner I. A lipase specific for esterified oxygenated polyenoic fatty acids in lipid bodies of cucumber cotyledons. In: Sánchez J, Cerdá-Olmedo E, Martínez-Force E, editors. Advances in plant lipid research. Sevilla: Secretariado de Publicaciones de la Universidad de Sevilla; 1998. pp. 320–322.
-
- Baud S, Dichow NR, Kelemen Z, et al. Regulation of HSD1 in seeds of Arabidopsis thaliana. Plant and Cell Physiology. 2009;50:1463–1478. - PubMed
-
- Behrends W, Rausch U, Löffler HG, Kindl H. Purification of glycolate oxidase from greening cucumber cotyledons. Planta. 1982;156:566–571. - PubMed
-
- Beisson F, Ferté N, Bruley S, Voultoury R, Verger R, Arondel V. Oil-bodies as substrates for lipolytic enzymes. Biochimica et Biophysica Acta. 2001;1531:47–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

