Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes
- PMID: 21081700
- PMCID: PMC3003076
- DOI: 10.1073/pnas.1015855107
Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes
Abstract
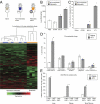
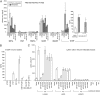
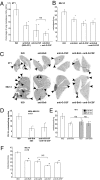
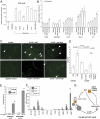
Priming of the organ-specific premetastatic sites is thought to be an important yet incompletely understood step during metastasis. In this study, we show that the metastatic tumors we examined overexpress granulocyte-colony stimulating factor (G-CSF), which expands and mobilizes Ly6G+Ly6C+ granulocytes and facilitates their subsequent homing at distant organs even before the arrival of tumor cells. Moreover, G-CSF-mobilized Ly6G+Ly6C+ cells produce the Bv8 protein, which has been implicated in angiogenesis and mobilization of myeloid cells. Anti-G-CSF or anti-Bv8 antibodies significantly reduced lung metastasis. Transplantation of Bv8 null fetal liver cells into lethally irradiated hosts also reduced metastasis. We identified an unexpected role for Bv8: the ability to stimulate tumor cell migration through activation of one of the Bv8 receptors, prokineticin receptor (PKR)-1. Finally, we show that administration of recombinant G-CSF is sufficient to increase the numbers of Ly6G+Ly6C+ cells in organ-specific metastatic sites and results in enhanced metastatic ability of several tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Nguyen DX, Bos PD, Massague J. Metastasis: From dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. - PubMed
-
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

