Calcium signaling by STIM and Orai: intimate coupling details revealed
- PMID: 21081752
- PMCID: PMC3601893
- DOI: 10.1126/scisignal.3148pe42
Calcium signaling by STIM and Orai: intimate coupling details revealed
Abstract
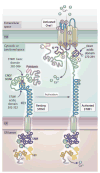
STIM (stromal interaction molecule) and Orai, two recently identified protein families, mediate cellular Ca(2+) signals through a remarkably dynamic interaction. STIM proteins are sensors of Ca(2+) stored within the endoplasmic reticulum (ER). Orai proteins are highly selective plasma membrane (PM) channels that allow only Ca(2+) ions to flow into cells. Although present in separate membranes, the two proteins undergo profound reorganization culminating in an exquisite pas de deux within small junctional regions between the ER and PM. Before these proteins can embrace, STIM undergoes an activation process triggered by depletion of Ca(2+) stores. During its union with Orai, STIM induces the channel pore within Orai to open, allowing Ca(2+) ions to flow through the PM and provide crucial intracellular signals. Recent studies on the activation of STIM and its coupling to Orai provide valuable new insights into the nature of the liaison between these two proteins and the intricate mechanism through which activation of Ca(2+) signals occurs.
Figures
Similar articles
-
STIM and Orai: dynamic intermembrane coupling to control cellular calcium signals.J Biol Chem. 2009 Aug 21;284(34):22501-5. doi: 10.1074/jbc.R109.018655. Epub 2009 May 27. J Biol Chem. 2009. PMID: 19473984 Free PMC article. Review.
-
The STIM-Orai Pathway: Conformational Coupling Between STIM and Orai in the Activation of Store-Operated Ca2+ Entry.Adv Exp Med Biol. 2017;993:83-98. doi: 10.1007/978-3-319-57732-6_5. Adv Exp Med Biol. 2017. PMID: 28900910 Free PMC article. Review.
-
The STIM-Orai coupling interface and gating of the Orai1 channel.Cell Calcium. 2017 May;63:8-13. doi: 10.1016/j.ceca.2017.01.001. Epub 2017 Jan 8. Cell Calcium. 2017. PMID: 28087079 Free PMC article. Review.
-
Orai channel C-terminal peptides are key modulators of STIM-Orai coupling and calcium signal generation.Cell Rep. 2021 Jun 29;35(13):109322. doi: 10.1016/j.celrep.2021.109322. Cell Rep. 2021. PMID: 34192542 Free PMC article.
-
Calcium store refilling and STIM activation in STIM- and Orai-deficient cell lines.Pflugers Arch. 2018 Oct;470(10):1555-1567. doi: 10.1007/s00424-018-2165-5. Epub 2018 Jun 22. Pflugers Arch. 2018. PMID: 29934936 Free PMC article.
Cited by
-
Hypoxia-induced acidosis uncouples the STIM-Orai calcium signaling complex.J Biol Chem. 2011 Dec 30;286(52):44788-98. doi: 10.1074/jbc.M111.303081. Epub 2011 Nov 14. J Biol Chem. 2011. PMID: 22084246 Free PMC article.
-
Store-operated calcium entry and diabetic complications.Exp Biol Med (Maywood). 2016 Feb;241(4):343-52. doi: 10.1177/1535370215609693. Epub 2015 Oct 14. Exp Biol Med (Maywood). 2016. PMID: 26468167 Free PMC article. Review.
-
Garcinol A Novel Inhibitor of Platelet Activation and Apoptosis.Toxins (Basel). 2019 Jul 1;11(7):382. doi: 10.3390/toxins11070382. Toxins (Basel). 2019. PMID: 31266175 Free PMC article.
-
Glucagon-like peptide-1 receptor pathway inhibits extracellular matrix production by mesangial cells through store-operated Ca2+ channel.Exp Biol Med (Maywood). 2019 Oct;244(14):1193-1201. doi: 10.1177/1535370219876531. Epub 2019 Sep 11. Exp Biol Med (Maywood). 2019. PMID: 31510798 Free PMC article.
-
STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation.EMBO J. 2011 May 4;30(9):1678-89. doi: 10.1038/emboj.2011.79. Epub 2011 Mar 22. EMBO J. 2011. PMID: 21427704 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

