Minicircle DNA-based gene therapy coupled with immune modulation permits long-term expression of α-L-iduronidase in mice with mucopolysaccharidosis type I
- PMID: 21081900
- PMCID: PMC3048178
- DOI: 10.1038/mt.2010.249
Minicircle DNA-based gene therapy coupled with immune modulation permits long-term expression of α-L-iduronidase in mice with mucopolysaccharidosis type I
Abstract
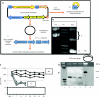
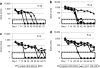
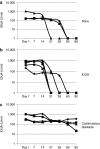
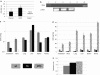
Mucopolysaccharidosis type I (MPS I) is a lysosomal storage disease characterized by mutations to the α-L-iduronidase (IDUA) gene resulting in inactivation of the IDUA enzyme. The loss of IDUA protein results in the progressive accumulation of glycosaminoglycans within the lysosomes resulting in severe, multi-organ system pathology. Gene replacement strategies have relied on the use of viral or nonviral gene delivery systems. Drawbacks to these include laborious production procedures, poor efficacy due to plasmid-borne gene silencing, and the risk of insertional mutagenesis. This report demonstrates the efficacy of a nonintegrating, minicircle (MC) DNA vector that is resistant to epigenetic gene silencing in vivo. To achieve sustained expression of the immunogenic IDUA protein we investigated the use of a tissue-specific promoter in conjunction with microRNA target sequences. The inclusion of microRNA target sequences resulted in a slight improvement in long-term expression compared to their absence. However, immune modulation by costimulatory blockade was required and permitted for IDUA expression in MPS I mice that resulted in the biochemical correction of pathology in all of the organs analyzed. MC gene delivery combined with costimulatory pathway blockade maximizes safety, efficacy, and sustained gene expression and is a new approach in the treatment of lysosomal storage disease.
Figures



Similar articles
-
Gene therapy for a mucopolysaccharidosis type I murine model with lentiviral-IDUA vector.Hum Gene Ther. 2005 Jan;16(1):81-90. doi: 10.1089/hum.2005.16.81. Hum Gene Ther. 2005. PMID: 15703491
-
Improvements in mucopolysaccharidosis I mice after adult retroviral vector-mediated gene therapy with immunomodulation.Mol Ther. 2007 May;15(5):889-902. doi: 10.1038/sj.mt.6300112. Epub 2007 Feb 20. Mol Ther. 2007. PMID: 17311010
-
α- L-iduronidase gene-based therapy using the phiC31 system to treat mucopolysaccharidose type I mice.J Gene Med. 2015 Jan-Feb;17(1-2):1-13. doi: 10.1002/jgm.2818. J Gene Med. 2015. PMID: 25597593
-
Mucopolysaccharidoses type I gene therapy.J Inherit Metab Dis. 2021 Sep;44(5):1088-1098. doi: 10.1002/jimd.12414. Epub 2021 Jul 9. J Inherit Metab Dis. 2021. PMID: 34189746 Free PMC article. Review.
-
Alpha-L-iduronidase and enzyme replacement therapy for mucopolysaccharidosis I.Expert Opin Biol Ther. 2002 Dec;2(8):967-76. doi: 10.1517/14712598.2.8.967. Expert Opin Biol Ther. 2002. PMID: 12517274 Review.
Cited by
-
Repair of Retinal Degeneration following Ex Vivo Minicircle DNA Gene Therapy and Transplantation of Corrected Photoreceptor Progenitors.Mol Ther. 2020 Mar 4;28(3):830-844. doi: 10.1016/j.ymthe.2020.01.023. Epub 2020 Jan 29. Mol Ther. 2020. PMID: 32027843 Free PMC article.
-
Intrinsic transgene immunogenicity gears CD8(+) T-cell priming after rAAV-mediated muscle gene transfer.Mol Ther. 2015 Apr;23(4):697-706. doi: 10.1038/mt.2014.235. Epub 2014 Dec 10. Mol Ther. 2015. PMID: 25492560 Free PMC article.
-
DNA vaccines for prostate cancer.Pharmacol Ther. 2017 Jun;174:27-42. doi: 10.1016/j.pharmthera.2017.02.016. Epub 2017 Feb 7. Pharmacol Ther. 2017. PMID: 28185916 Free PMC article. Review.
-
Gene therapy for neurologic manifestations of mucopolysaccharidoses.Expert Opin Drug Deliv. 2015 Feb;12(2):283-96. doi: 10.1517/17425247.2015.966682. Epub 2014 Dec 16. Expert Opin Drug Deliv. 2015. PMID: 25510418 Free PMC article. Review.
-
Angiotensin-converting enzyme 2 amplification limited to the circulation does not protect mice from development of diabetic nephropathy.Kidney Int. 2017 Jun;91(6):1336-1346. doi: 10.1016/j.kint.2016.09.032. Epub 2016 Dec 4. Kidney Int. 2017. PMID: 27927599 Free PMC article.
References
-
- Chen ZY, Riu E, He CY, Xu H., and, Kay MA. Silencing of episomal transgene expression in liver by plasmid bacterial backbone DNA is independent of CpG methylation. Mol Ther. 2008;16:548–556. - PubMed
-
- Riu E, Grimm D, Huang Z., and, Kay MA. Increased maintenance and persistence of transgenes by excision of expression cassettes from plasmid sequences in vivo. Hum Gene Ther. 2005;16:558–570. - PubMed
-
- Thomas CE, Ehrhardt A., and, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. - PubMed
-
- Yew NS., and, Cheng SH. Reducing the immunostimulatory activity of CpG-containing plasmid DNA vectors for non-viral gene therapy. Expert Opin Drug Deliv. 2004;1:115–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

