Pentalenic acid is a shunt metabolite in the biosynthesis of the pentalenolactone family of metabolites: hydroxylation of 1-deoxypentalenic acid mediated by CYP105D7 (SAV_7469) of Streptomyces avermitilis
- PMID: 21081950
- PMCID: PMC3030646
- DOI: 10.1038/ja.2010.135
Pentalenic acid is a shunt metabolite in the biosynthesis of the pentalenolactone family of metabolites: hydroxylation of 1-deoxypentalenic acid mediated by CYP105D7 (SAV_7469) of Streptomyces avermitilis
Abstract
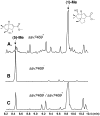
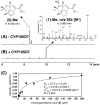
Pentalenic acid (1) has been isolated from many Streptomyces sp. as a co-metabolite of the sesquiterpenoid antibiotic pentalenolactone and related natural products. We have previously reported the identification of a 13.4-kb gene cluster in the genome of Streptomyces avermitilis implicated in the biosynthesis of the pentalenolactone family of metabolites consisting of 13 open reading frames. Detailed molecular genetic and biochemical studies have revealed that at least seven genes are involved in the biosynthesis of the newly discovered metabolites, neopentalenoketolactone, but no gene specifically dedicated to the formation of pentalenic acid (1) was evident in the same gene cluster. The wild-type strain of S. avermitilis, as well as its derivatives, mainly produce pentalenic acid (1), together with neopentalenoketolactone (9). Disruption of the sav7469 gene encoding a cytochrome P450 (CYP105D7), members of which class are associated with the hydroxylation of many structurally different compounds, abolished the production of pentalenic acid (1). The sav7469-deletion mutant derived from SUKA11 carrying pKU462∷ptl-clusterΔptlH accumulated 1-deoxypentalenic acid (5), but not pentalenic acid (1). Reintroduction of an extra-copy of the sav7469 gene to SUKA11 Δsav7469 carrying pKU462∷ptl-clusterΔptlH restored the production of pentalenic acid (1). Recombinant CYP105D7 prepared from Escherichia coli catalyzed the oxidative conversion of 1-deoxypentalenic acid (5) to pentalenic acid (1) in the presence of the electron-transport partners, ferredoxin (Fdx) and Fdx reductase, both in vivo and in vitro. These results unambiguously demonstrate that CYP105D7 is responsible for the conversion of 1-deoxypentalenic acid (5) to pentalenic acid (1), a shunt product in the biosynthesis of the pentalenolactone family of metabolites.
Figures
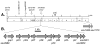



Similar articles
-
A gene cluster for biosynthesis of the sesquiterpenoid antibiotic pentalenolactone in Streptomyces avermitilis.Biochemistry. 2006 May 16;45(19):6179-86. doi: 10.1021/bi060419n. Biochemistry. 2006. PMID: 16681390 Free PMC article.
-
Hydroxylation of Compactin (ML-236B) by CYP105D7 (SAV_7469) from Streptomyces avermitilis.J Microbiol Biotechnol. 2017 May 28;27(5):956-964. doi: 10.4014/jmb.1610.10079. J Microbiol Biotechnol. 2017. PMID: 28274099
-
Regioselective hydroxylation of daidzein using P450 (CYP105D7) from Streptomyces avermitilis MA4680.Biotechnol Bioeng. 2010 Mar 1;105(4):697-704. doi: 10.1002/bit.22582. Biotechnol Bioeng. 2010. PMID: 19845003
-
Pentalenolactone biosynthesis. Molecular cloning and assignment of biochemical function to PtlI, a cytochrome P450 of Streptomyces avermitilis.J Am Chem Soc. 2006 Oct 11;128(40):13036-7. doi: 10.1021/ja0639214. J Am Chem Soc. 2006. PMID: 17017767 Free PMC article.
-
Effect of Extracellular Tyrosinase on the Expression Level of P450, Fpr, and Fdx and Ortho-hydroxylation of Daidzein in Streptomyces avermitilis.Appl Biochem Biotechnol. 2018 Mar;184(3):1036-1046. doi: 10.1007/s12010-017-2606-1. Epub 2017 Sep 22. Appl Biochem Biotechnol. 2018. PMID: 28940109
Cited by
-
Genome mining of the Streptomyces avermitilis genome and development of genome-minimized hosts for heterologous expression of biosynthetic gene clusters.J Ind Microbiol Biotechnol. 2014 Feb;41(2):233-50. doi: 10.1007/s10295-013-1327-x. Epub 2013 Aug 29. J Ind Microbiol Biotechnol. 2014. PMID: 23990133 Review.
-
Genome mining in Streptomyces. Elucidation of the role of Baeyer-Villiger monooxygenases and non-heme iron-dependent dehydrogenase/oxygenases in the final steps of the biosynthesis of pentalenolactone and neopentalenolactone.Biochemistry. 2011 Mar 15;50(10):1739-54. doi: 10.1021/bi1019786. Epub 2011 Feb 8. Biochemistry. 2011. PMID: 21250661 Free PMC article.
-
CYP105-diverse structures, functions and roles in an intriguing family of enzymes in Streptomyces.J Appl Microbiol. 2014 Dec;117(6):1549-63. doi: 10.1111/jam.12662. Epub 2014 Nov 4. J Appl Microbiol. 2014. PMID: 25294646 Free PMC article. Review.
-
Actinomycetes as Producers of Biologically Active Terpenoids: Current Trends and Patents.Pharmaceuticals (Basel). 2023 Jun 12;16(6):872. doi: 10.3390/ph16060872. Pharmaceuticals (Basel). 2023. PMID: 37375819 Free PMC article. Review.
-
Genome mining in streptomyces. Discovery of an unprecedented P450-catalyzed oxidative rearrangement that is the final step in the biosynthesis of pentalenolactone.J Am Chem Soc. 2011 Feb 23;133(7):2128-31. doi: 10.1021/ja111279h. Epub 2011 Feb 1. J Am Chem Soc. 2011. PMID: 21284395 Free PMC article.
References
-
- Koe BK, Sobin BA, Celmer WD. PA 132, a New Antibiotic. I. Isolation and Chemical Properties. Antibiot Annu. 1957:672–675. - PubMed
-
- Martin DG, Slomp G, Mizsak S, Duchamp DJ, Chidester CG. The Structure and Absolute Configuration of Pentalenolactone (PA 132) Tetrahedron Lett. 1970;11:4901–4904. - PubMed
-
- Keller-Schierlein W, Lemke J, Nyfeler R, Zähner H. Stoffwechelprodukte von Mikroorganismen. 105. Arenaemycin E, D, und C. Arch Mikrobiol. 1972;84:301–316. - PubMed
-
- Takahashi S, Takeuchi M, Arai M, Seto H, Otake N. Studies on the Biosynthesis of Pentalenolactone. V. Isolation of Deoxypentalenylglucuron. J Antibiot. 1983;36:226–228. - PubMed
-
- English AR, McBride TJ, Lynch JE. PA 132, A New Antibiotic. II. In Vitro and in Vivo Studies. Antibiot Annu. 1957:682–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

