Pentalenic acid is a shunt metabolite in the biosynthesis of the pentalenolactone family of metabolites: hydroxylation of 1-deoxypentalenic acid mediated by CYP105D7 (SAV_7469) of Streptomyces avermitilis
- PMID: 21081950
- PMCID: PMC3030646
- DOI: 10.1038/ja.2010.135
Pentalenic acid is a shunt metabolite in the biosynthesis of the pentalenolactone family of metabolites: hydroxylation of 1-deoxypentalenic acid mediated by CYP105D7 (SAV_7469) of Streptomyces avermitilis
Abstract
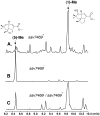
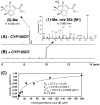
Pentalenic acid (1) has been isolated from many Streptomyces sp. as a co-metabolite of the sesquiterpenoid antibiotic pentalenolactone and related natural products. We have previously reported the identification of a 13.4-kb gene cluster in the genome of Streptomyces avermitilis implicated in the biosynthesis of the pentalenolactone family of metabolites consisting of 13 open reading frames. Detailed molecular genetic and biochemical studies have revealed that at least seven genes are involved in the biosynthesis of the newly discovered metabolites, neopentalenoketolactone, but no gene specifically dedicated to the formation of pentalenic acid (1) was evident in the same gene cluster. The wild-type strain of S. avermitilis, as well as its derivatives, mainly produce pentalenic acid (1), together with neopentalenoketolactone (9). Disruption of the sav7469 gene encoding a cytochrome P450 (CYP105D7), members of which class are associated with the hydroxylation of many structurally different compounds, abolished the production of pentalenic acid (1). The sav7469-deletion mutant derived from SUKA11 carrying pKU462∷ptl-clusterΔptlH accumulated 1-deoxypentalenic acid (5), but not pentalenic acid (1). Reintroduction of an extra-copy of the sav7469 gene to SUKA11 Δsav7469 carrying pKU462∷ptl-clusterΔptlH restored the production of pentalenic acid (1). Recombinant CYP105D7 prepared from Escherichia coli catalyzed the oxidative conversion of 1-deoxypentalenic acid (5) to pentalenic acid (1) in the presence of the electron-transport partners, ferredoxin (Fdx) and Fdx reductase, both in vivo and in vitro. These results unambiguously demonstrate that CYP105D7 is responsible for the conversion of 1-deoxypentalenic acid (5) to pentalenic acid (1), a shunt product in the biosynthesis of the pentalenolactone family of metabolites.
Figures



References
-
- Koe BK, Sobin BA, Celmer WD. PA 132, a New Antibiotic. I. Isolation and Chemical Properties. Antibiot Annu. 1957:672–675. - PubMed
-
- Martin DG, Slomp G, Mizsak S, Duchamp DJ, Chidester CG. The Structure and Absolute Configuration of Pentalenolactone (PA 132) Tetrahedron Lett. 1970;11:4901–4904. - PubMed
-
- Keller-Schierlein W, Lemke J, Nyfeler R, Zähner H. Stoffwechelprodukte von Mikroorganismen. 105. Arenaemycin E, D, und C. Arch Mikrobiol. 1972;84:301–316. - PubMed
-
- Takahashi S, Takeuchi M, Arai M, Seto H, Otake N. Studies on the Biosynthesis of Pentalenolactone. V. Isolation of Deoxypentalenylglucuron. J Antibiot. 1983;36:226–228. - PubMed
-
- English AR, McBride TJ, Lynch JE. PA 132, A New Antibiotic. II. In Vitro and in Vivo Studies. Antibiot Annu. 1957:682–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

