Expression and membrane topology of Anopheles gambiae odorant receptors in lepidopteran insect cells
- PMID: 21082026
- PMCID: PMC2972716
- DOI: 10.1371/journal.pone.0015428
Expression and membrane topology of Anopheles gambiae odorant receptors in lepidopteran insect cells
Abstract
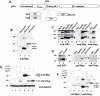
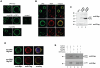
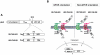
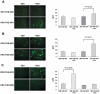
A lepidopteran insect cell-based expression system has been employed to express three Anopheles gambiae odorant receptors (ORs), OR1 and OR2, which respond to components of human sweat, and OR7, the ortholog of Drosophila's OR83b, the heteromerization partner of all functional ORs in that system. With the aid of epitope tagging and specific antibodies, efficient expression of all ORs was demonstrated and intrinsic properties of the proteins were revealed. Moreover, analysis of the orientation of OR1 and OR2 on the cellular plasma membrane through the use of a novel 'topology screen' assay and FACS analysis demonstrates that, as was recently reported for the ORs in Drosophila melanogaster, mosquito ORs also have a topology different than their mammalian counterparts with their N-terminal ends located in the cytoplasm and their C-terminal ends facing outside the cell. These results set the stage for the production of mosquito ORs in quantities that should permit their detailed biochemical and structural characterization and the exploration of their functional properties.
Conflict of interest statement
Figures
Similar articles
-
Odorant Binding Proteins (OBPs) and Odorant Receptors (ORs) of Anopheles stephensi: Identification and comparative insights.PLoS One. 2022 Mar 22;17(3):e0265896. doi: 10.1371/journal.pone.0265896. eCollection 2022. PLoS One. 2022. PMID: 35316281 Free PMC article.
-
Blockade of insect odorant receptor currents by amiloride derivatives.Chem Senses. 2013 Mar;38(3):221-9. doi: 10.1093/chemse/bjs100. Epub 2013 Jan 4. Chem Senses. 2013. PMID: 23292750 Free PMC article.
-
Identification and expression of odorant-binding proteins of the malaria-carrying mosquitoes Anopheles gambiae and Anopheles arabiensis.Arch Insect Biochem Physiol. 2005 Mar;58(3):175-89. doi: 10.1002/arch.20047. Arch Insect Biochem Physiol. 2005. PMID: 15717318
-
G protein-coupled receptors in Anopheles gambiae.Science. 2002 Oct 4;298(5591):176-8. doi: 10.1126/science.1076196. Science. 2002. PMID: 12364795
-
The 40-Year Mystery of Insect Odorant-Binding Proteins.Biomolecules. 2021 Mar 30;11(4):509. doi: 10.3390/biom11040509. Biomolecules. 2021. PMID: 33808208 Free PMC article. Review.
Cited by
-
Inhibition of Anopheles gambiae odorant receptor function by mosquito repellents.J Biol Chem. 2015 Mar 20;290(12):7961-72. doi: 10.1074/jbc.M114.632299. Epub 2015 Feb 5. J Biol Chem. 2015. PMID: 25657000 Free PMC article.
-
Odorant Receptors and Odorant-Binding Proteins as Insect Pest Control Targets: A Comparative Analysis.Front Physiol. 2018 Aug 24;9:1163. doi: 10.3389/fphys.2018.01163. eCollection 2018. Front Physiol. 2018. PMID: 30197600 Free PMC article. Review.
-
Antennal expression pattern of two olfactory receptors and an odorant binding protein implicated in host odor detection by the malaria vector Anopheles gambiae.Int J Biol Sci. 2010 Oct 8;6(7):614-26. doi: 10.7150/ijbs.6.614. Int J Biol Sci. 2010. PMID: 20975820 Free PMC article.
-
Transcuticular calcium imaging as a tool for the functional study of insect odorant receptors.Front Mol Neurosci. 2023 Aug 14;16:1182361. doi: 10.3389/fnmol.2023.1182361. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37645702 Free PMC article.
-
Mass Spectrometry-Based Screening Platform Reveals Orco Interactome in Drosophila melanogaster.Mol Cells. 2018 Feb 28;41(2):150-159. doi: 10.14348/molcells.2018.2305. Epub 2018 Feb 12. Mol Cells. 2018. PMID: 29429152 Free PMC article.
References
-
- Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

