Localization and androgen regulation of metastasis-associated protein 1 in mouse epididymis
- PMID: 21082030
- PMCID: PMC2972736
- DOI: 10.1371/journal.pone.0015439
Localization and androgen regulation of metastasis-associated protein 1 in mouse epididymis
Abstract
Background: Metastasis-associated protein 1 (MTA1), the founding member of the MTA family of genes, can modulate transcription by influencing the status of chromatin remodeling. Despite its strong correlation with the metastatic potential of cancer cells, MTA1 can also regulate crucial cellular pathways by modifying the acetylation status. We have previously reported the presence of MTA1/MTA1 in human and mouse testes, providing the evidence for its involvement in the regulation of testicular function during murine spermatogenesis. The objective of present study was to further assess the localization of MTA1 in mouse epididymis on both transcriptional and translational level, and then to explore whether MTA1 expression is regulated by androgens and postnatal epididymal development.
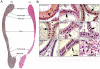
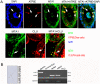
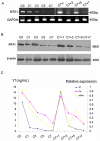
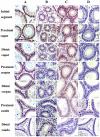
Methodology/principal findings: Mice were deprived of circulating androgen by bilaterally castration and were then supplemented with exogenous testosterone propionate for one week. MTA1 was immunolocalized in the epithelium of the entire epididymis with the maximal expression in the nuclei of principal cells and of clear cells in proximal region. Its expression decreased gradually after castration, whereas testosterone treatment could restore the expression, indicating that the expression of this gene is dependent on androgen. During postnatal development, the protein expression in the epididymis began to appear from day 7 to day 14, increased dramatically from postnatal day 28, and peaked at adulthood onwards, coinciding with both the well differentiated status of epididymis and the mature levels of circulating androgens. This region- and cell-specific pattern was also conservative in normal human epididymis.
Conclusions: Our data suggest that the expression of MTA1 protein could be regulated by androgen pathway and its expression level is closely associated with the postnatal development of the epididymis, giving rise to the possibility that this gene plays a potential role in sperm maturation and fertility.
Conflict of interest statement
Figures



Similar articles
-
Sperm-associated antigen 11A is expressed exclusively in the principal cells of the mouse caput epididymis in an androgen-dependent manner.Reprod Biol Endocrinol. 2013 Jul 1;11:59. doi: 10.1186/1477-7827-11-59. Reprod Biol Endocrinol. 2013. PMID: 23815807 Free PMC article.
-
Postnatal expression and androgen regulation of HOXBES2 homeoprotein in rat epididymis.J Androl. 2007 Sep-Oct;28(5):755-71. doi: 10.2164/jandrol.106.002394. Epub 2007 May 9. J Androl. 2007. PMID: 17494099
-
Androgenic regulation of beta-defensins in the mouse epididymis.Reprod Biol Endocrinol. 2014 Aug 7;12:76. doi: 10.1186/1477-7827-12-76. Reprod Biol Endocrinol. 2014. PMID: 25099571 Free PMC article.
-
The role of the MTA family and their encoded proteins in human cancers: molecular functions and clinical implications.Clin Exp Metastasis. 2009;26(3):215-27. doi: 10.1007/s10585-008-9233-8. Epub 2008 Dec 31. Clin Exp Metastasis. 2009. PMID: 19116762 Review.
-
Emerging roles of MTA family members in human cancers.Semin Oncol. 2003 Oct;30(5 Suppl 16):30-7. doi: 10.1053/j.seminoncol.2003.08.005. Semin Oncol. 2003. PMID: 14613024 Review.
Cited by
-
Transient protection from heat-stress induced apoptotic stimulation by metastasis-associated protein 1 in pachytene spermatocytes.PLoS One. 2011;6(10):e26013. doi: 10.1371/journal.pone.0026013. Epub 2011 Oct 12. PLoS One. 2011. PMID: 22022494 Free PMC article.
-
Organic Zinc and Copper Supplementation-Associated Changes in Gene Expression and Protein Profiles in Buck Spermatozoa.Biol Trace Elem Res. 2022 Apr;200(4):1626-1639. doi: 10.1007/s12011-021-02796-x. Epub 2021 Jul 8. Biol Trace Elem Res. 2022. PMID: 34235611
-
Distinct actions of testicular endocrine and lumicrine signaling on the proximal epididymal transcriptome.Reprod Biol Endocrinol. 2024 Apr 10;22(1):40. doi: 10.1186/s12958-024-01213-x. Reprod Biol Endocrinol. 2024. PMID: 38600586 Free PMC article.
-
Androgens Enhance Adult Hippocampal Neurogenesis in Males but Not Females in an Age-Dependent Manner.Endocrinology. 2019 Sep 1;160(9):2128-2136. doi: 10.1210/en.2019-00114. Endocrinology. 2019. PMID: 31219567 Free PMC article.
-
Sertoli cell-specific expression of metastasis-associated protein 2 (MTA2) is required for transcriptional regulation of the follicle-stimulating hormone receptor (FSHR) gene during spermatogenesis.J Biol Chem. 2012 Nov 23;287(48):40471-83. doi: 10.1074/jbc.M112.383802. Epub 2012 Oct 18. J Biol Chem. 2012. PMID: 23086931 Free PMC article.
References
-
- Robaire B, Seenundun S, Hamzeh M, Lamour SA. Androgenic regulation of novel genes in the epididymis. Asian J Androl. 2007;9:545–553. - PubMed
-
- Chauvin TR, Griswold MD. Androgen-regulated genes in the murine epididymis. Biol Reprod. 2004;71:560–569. - PubMed
-
- Prabagaran E, Hegde UC, Moodbidri SB, Bandivdekar AH, Raghavan VP. Postnatal expression and androgen regulation of HOXBES2 homeoprotein in rat epididymis. J Androl. 2007;28:755–771. - PubMed
-
- Baska KM, Manandhar G, Feng D, Agca Y, Tengowski MW, et al. Mechanism of extracellular ubiquitination in the mammalian epididymis. J Cell Physiol. 2008;215:684–696. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

