Disentangling the functional roles of morphology and motion in the swimming of fish
- PMID: 21082068
- PMCID: PMC2981590
- DOI: 10.1093/icb/icq057
Disentangling the functional roles of morphology and motion in the swimming of fish
Abstract
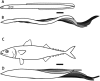
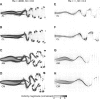
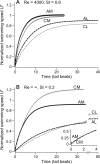
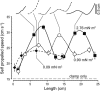
In fishes the shape of the body and the swimming mode generally are correlated. Slender-bodied fishes such as eels, lampreys, and many sharks tend to swim in the anguilliform mode, in which much of the body undulates at high amplitude. Fishes with broad tails and a narrow caudal peduncle, in contrast, tend to swim in the carangiform mode, in which the tail undulates at high amplitude. Such fishes also tend to have different wake structures. Carangiform swimmers generally produce two staggered vortices per tail beat and a strong downstream jet, while anguilliform swimmers produce a more complex wake, containing at least two pairs of vortices per tail beat and relatively little downstream flow. Are these differences a result of the different swimming modes or of the different body shapes, or both? Disentangling the functional roles requires a multipronged approach, using experiments on live fishes as well as computational simulations and physical models. We present experimental results from swimming eels (anguilliform), bluegill sunfish (carangiform), and rainbow trout (subcarangiform) that demonstrate differences in the wakes and in swimming performance. The swimming of mackerel and lamprey was also simulated computationally with realistic body shapes and both swimming modes: the normal carangiform mackerel and anguilliform lamprey, then an anguilliform mackerel and carangiform lamprey. The gross structure of simulated wakes (single versus double vortex row) depended strongly on Strouhal number, while body shape influenced the complexity of the vortex row, and the swimming mode had the weakest effect. Performance was affected even by small differences in the wakes: both experimental and computational results indicate that anguilliform swimmers are more efficient at lower swimming speeds, while carangiform swimmers are more efficient at high speed. At high Reynolds number, the lamprey-shaped swimmer produced a more complex wake than the mackerel-shaped swimmer, similar to the experimental results. Finally, we show results from a simple physical model of a flapping fin, using fins of different flexural stiffness. When actuated in the same way, fins of different stiffnesses propel themselves at different speeds with different kinematics. Future experimental and computational work will need to consider the mechanisms underlying production of the anguilliform and carangiform swimming modes, because anguilliform swimmers tend to be less stiff, in general, than are carangiform swimmers.
Figures





Similar articles
-
On the role of form and kinematics on the hydrodynamics of self-propelled body/caudal fin swimming.J Exp Biol. 2010 Jan 1;213(1):89-107. doi: 10.1242/jeb.030932. J Exp Biol. 2010. PMID: 20008366
-
Numerical investigation of the hydrodynamics of anguilliform swimming in the transitional and inertial flow regimes.J Exp Biol. 2009 Feb;212(Pt 4):576-92. doi: 10.1242/jeb.025007. J Exp Biol. 2009. PMID: 19181905
-
Convergence of undulatory swimming kinematics across a diversity of fishes.Proc Natl Acad Sci U S A. 2021 Dec 7;118(49):e2113206118. doi: 10.1073/pnas.2113206118. Proc Natl Acad Sci U S A. 2021. PMID: 34853171 Free PMC article.
-
Hydrodynamics of C-Start Escape Responses of Fish as Studied with Simple Physical Models.Integr Comp Biol. 2015 Oct;55(4):728-39. doi: 10.1093/icb/icv016. Epub 2015 Apr 27. Integr Comp Biol. 2015. PMID: 25920507 Review.
-
Form and function of anguilliform swimming.Biol Rev Camb Philos Soc. 2024 Dec;99(6):2190-2210. doi: 10.1111/brv.13116. Epub 2024 Jul 14. Biol Rev Camb Philos Soc. 2024. PMID: 39004428 Review.
Cited by
-
Larval zebrafish maintain elevation with multisensory control of posture and locomotion.bioRxiv [Preprint]. 2025 Aug 2:2024.01.23.576760. doi: 10.1101/2024.01.23.576760. bioRxiv. 2025. Update in: J Exp Biol. 2025 Aug 07:jeb.250087. doi: 10.1242/jeb.250087. PMID: 38328242 Free PMC article. Updated. Preprint.
-
Body fineness ratio as a predictor of maximum prolonged-swimming speed in coral reef fishes.PLoS One. 2013 Oct 18;8(10):e75422. doi: 10.1371/journal.pone.0075422. eCollection 2013. PLoS One. 2013. PMID: 24204575 Free PMC article.
-
Three-dimensional scaling laws of cetacean propulsion characterize the hydrodynamic interplay of flukes' shape and kinematics.J R Soc Interface. 2020 Feb;17(163):20190655. doi: 10.1098/rsif.2019.0655. Epub 2020 Feb 26. J R Soc Interface. 2020. PMID: 32093541 Free PMC article.
-
Energy efficiency and allometry of movement of swimming and flying animals.Proc Natl Acad Sci U S A. 2014 May 27;111(21):7517-21. doi: 10.1073/pnas.1310544111. Epub 2014 May 12. Proc Natl Acad Sci U S A. 2014. PMID: 24821764 Free PMC article.
-
The effects of warm thermal variability on metabolism and swimming performance in wild Atlantic salmon (Salmo salar).J Fish Biol. 2025 Mar;106(3):893-907. doi: 10.1111/jfb.15996. Epub 2024 Nov 24. J Fish Biol. 2025. PMID: 39581221 Free PMC article.
References
-
- Alben S. Optimal flexibility of a flapping appendage in an inviscid fluid. J Fluid Mech. 2008;614:355–380.
-
- Aleyev YG. Nekton. The Hague: Junk; 1977.
-
- Anderson J. Vorticity control for efficient propulsion. PhD Thesis. Cambridge (MA): MIT/WHOI; 1996. pp. 96–02.
-
- Ayali A, Gelman S, Tytell ED, Cohen AH. Lateral line activity during swimming-like motion suggests a feedback link in closed-loop control of lamprey swimming. Can J Zool. 2009;87:671–83.
-
- Ayers J. Recovery of oscillator function following spinal regeneration in the sea lamprey. In: Jacklet J, editor. Cellular and Neuronal Oscillators. New York: Marcel Dekker; 1989. pp. 349–83.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

