Multiparametric magnetic resonance imaging and repeated measurements of blood-brain barrier permeability to contrast agents
- PMID: 21082372
- PMCID: PMC3788825
- DOI: 10.1007/978-1-60761-938-3_8
Multiparametric magnetic resonance imaging and repeated measurements of blood-brain barrier permeability to contrast agents
Abstract
Breakdown of the blood-brain barrier (BBB) is present in several neurological disorders such as stroke, brain tumors, and multiple sclerosis. Noninvasive evaluation of BBB breakdown is important for monitoring disease progression and evaluating therapeutic efficacy in such disorders. One of the few techniques available for noninvasively and repeatedly localizing and quantifying BBB damage is magnetic resonance imaging (MRI). This usually involves the intravenous administration of a gadolinium-containing MR contrast agent (MRCA) such as Gadolinium-diethylenetriaminepentaacetic acid (Gd-DTPA), followed by dynamic contrast-enhanced MR imaging (DCE-MRI) of brain and blood, and analysis of the resultant data to derive indices of blood-to-brain transfer. There are two advantages to this approach. First, measurements can be made repeatedly in the same animal; for instance, they can be made before drug treatment and then again after treatment to assess efficacy. Secondly, MRI studies can be multiparametric. That is, MRI can be used to assess not only a blood-to-brain transfer or influx rate constant (Ki or K1) by DCE-MRI but also complementary parameters such as: (1) cerebral blood flow (CBF), done in our hands by arterial spin-tagging (AST) methods; (2) magnetization transfer (MT) parameters, most notably T1sat, which appear to reflect brain water-protein interactions plus BBB and tissue dysfunction; (3) the apparent diffusion coefficient of water (ADCw) and/or diffusion tensor, which is a function of the size and tortuosity of the extracellular space; and (4) the transverse relaxation time by T2-weighted imaging, which demarcates areas of tissue abnormality in many cases. The accuracy and reliability of two of these multiparametric MRI measures, CBF by AST and DCE-MRI determined influx of Gd-DTPA, have been established by nearly congruent quantitative autoradiographic (QAR) studies with appropriate radiotracers. In addition, some of their linkages to local pathology have been shown via corresponding light microscopy and fluorescence imaging. This chapter describes: (1) multiparametric MRI techniques with emphasis on DCE-MRI and AST-MRI; (2) the measurement of the blood-to-brain influx rate constant and CBF; and (3) the role of each in determining BBB permeability.
Figures
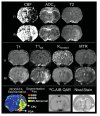
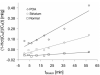


References
-
- Fenstermacher JD, Nagaraja T, Davies KR. In: Overview of the structure and function of the blood-brain barrier in vivo. Kobiler SL D, Shapira S, editors. Kluwer Academic/Plenum Publishers; New York: 2001. pp. 1–7.
-
- Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. - PubMed
-
- Knight RA, Barker PB, Fagan SC, Li Y, Jacobs MA, Welch KM. Prediction of impending hemorrhagic transformation in ischemic stroke using magnetic resonance imaging in rats. Stroke. 1998;29:144–151. - PubMed
-
- The NINDS. (The NINDS t-PA Stroke Study Group) Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. - PubMed
-
- Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, Bammer R, Kakuda W, Lansberg MG, Shuaib A, Coplin W, Hamilton S, Moseley M, Marks MP. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

