Advancing the mechanistic understanding of an enantioselective palladium-catalyzed alkene difunctionalization reaction
- PMID: 21082845
- PMCID: PMC3066298
- DOI: 10.1021/ja108106h
Advancing the mechanistic understanding of an enantioselective palladium-catalyzed alkene difunctionalization reaction
Abstract
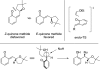
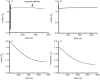
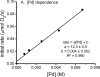
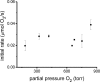
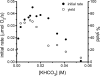
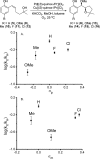
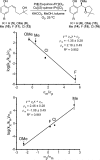
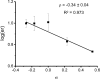
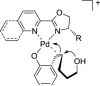
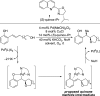
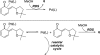
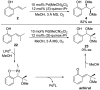
The mechanism of an enantioselective palladium-catalyzed alkene difunctionalization reaction has been investigated. Kinetic analysis provides evidence of turnover-limiting attack of a proposed quinone methide intermediate with MeOH and suggests that copper is involved in productive product formation, not just catalyst turnover. Through examination of substrate electronic effects, a Jaffé relationship was observed correlating rate to electronic perturbation at two positions of the substrate. Ligand effects were evaluated to provide evidence of rapid ligand exchange between palladium and copper as well as a correlation between ligand electronic nature and enantioselectivity.
Figures
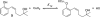
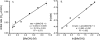
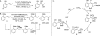

References
-
- Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483.
-
- Cardona F, Goti A. Nat. Chem. 2009;1:269. - PubMed
-
- Bäckvall J-E. Met.-Catal. Cross-Coupling React. (2nd Ed.) 2004;2:479.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

