Mechanisms of amine accumulation in, and egress from, lysosomes
- PMID: 21083094
- PMCID: PMC3065188
- DOI: 10.4155/bio.09.128
Mechanisms of amine accumulation in, and egress from, lysosomes
Abstract
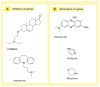
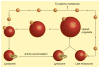
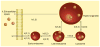
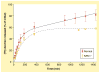
The human body is continuously exposed to small organic molecules containing one or more basic nitrogen atoms. Many of these are endogenous (i.e., neurotransmitters, polyamines and biogenic amines), while others are exogenously supplied in the form of drugs, foods and pollutants. It is well-known that many amines have a strong propensity to specifically and substantially accumulate in highly acidic intracellular compartments, such as lysosomes, through a mechanism referred to as ion trapping. It is also known that cells have acquired the unique ability to sense and respond to amine accumulation in lysosomes in an effort to prevent potential negative consequences associated with hyperaccumulation. We describe here methods that are used to evaluate the dynamics of amine accumulation in, and egress from, lysosomes. Moreover, we highlight specific proteins that are thought to play important roles in these pathways. A theoretical model describing lysosomal amine dynamics is described and shown to adequately fit experimental kinetic data. The implications of this research in understanding and treating disease are discussed.
Figures





Similar articles
-
Lysosomal sequestration of amine-containing drugs: analysis and therapeutic implications.J Pharm Sci. 2007 Apr;96(4):729-46. doi: 10.1002/jps.20792. J Pharm Sci. 2007. PMID: 17117426 Review.
-
Time-dependent effects of hydrophobic amine-containing drugs on lysosome structure and biogenesis in cultured human fibroblasts.J Pharm Sci. 2014 Oct;103(10):3287-96. doi: 10.1002/jps.24087. Epub 2014 Jul 16. J Pharm Sci. 2014. PMID: 25042198
-
Cationic amphiphilic drugs cause a marked expansion of apparent lysosomal volume: implications for an intracellular distribution-based drug interaction.Mol Pharm. 2012 May 7;9(5):1384-95. doi: 10.1021/mp200641e. Epub 2012 Apr 6. Mol Pharm. 2012. PMID: 22449202 Free PMC article.
-
Lysosomal sequestration (trapping) of lipophilic amine (cationic amphiphilic) drugs in immortalized human hepatocytes (Fa2N-4 cells).Drug Metab Dispos. 2013 Apr;41(4):897-905. doi: 10.1124/dmd.112.050054. Epub 2013 Feb 1. Drug Metab Dispos. 2013. PMID: 23378628 Free PMC article.
-
Drug-drug interactions involving lysosomes: mechanisms and potential clinical implications.Expert Opin Drug Metab Toxicol. 2012 Aug;8(8):943-58. doi: 10.1517/17425255.2012.691165. Epub 2012 May 22. Expert Opin Drug Metab Toxicol. 2012. PMID: 22616667 Review.
Cited by
-
Structural basis of the anti-ageing effects of polyphenolics: mitigation of oxidative stress.BMC Chem. 2020 Aug 10;14(1):50. doi: 10.1186/s13065-020-00696-0. eCollection 2020 Dec. BMC Chem. 2020. PMID: 32793891 Free PMC article. Review.
-
The Development of LAT1 Efflux Agonists as Mechanistic Probes of Cellular Amino Acid Stress.Biomolecules. 2024 Mar 9;14(3):326. doi: 10.3390/biom14030326. Biomolecules. 2024. PMID: 38540746 Free PMC article.
-
Stabilin-1 and Stabilin-2 are specific receptors for the cellular internalization of phosphorothioate-modified antisense oligonucleotides (ASOs) in the liver.Nucleic Acids Res. 2016 Apr 7;44(6):2782-94. doi: 10.1093/nar/gkw112. Epub 2016 Feb 22. Nucleic Acids Res. 2016. PMID: 26908652 Free PMC article.
-
Triarylpyridine Compounds and Chloroquine Act in Concert to Trigger Lysosomal Membrane Permeabilization and Cell Death in Cancer Cells.Cancers (Basel). 2020 Jun 18;12(6):1621. doi: 10.3390/cancers12061621. Cancers (Basel). 2020. PMID: 32570977 Free PMC article.
-
Repurposing Sigma-1 Receptor Ligands for COVID-19 Therapy?Front Pharmacol. 2020 Nov 9;11:582310. doi: 10.3389/fphar.2020.582310. eCollection 2020. Front Pharmacol. 2020. PMID: 33364957 Free PMC article. Review.
References
-
- Coffey JW, de Duve C. Digestive activity of lysosomes. I. The digestion of proteins by extracts of rat liver lysosomes. J Biol Chem. 1968;243:3255–3263. - PubMed
-
- de Duve C, De Barsy T, Poole B, Trouet A, Tulkens P, Van Hoof F. Lysosomotropic agents. Biochem Pharmacol. 1974;23(18):2495–2531. Comprehensive review into lysosomotropism, including discussions on lysosomes, pH partitioning, drug accumulation and disease states. - PubMed
-
- Fowler S, de Duve C. Digestive activity of lysosomes. 3 The digestion of lipids by extracts of rat liver lysosomes. J Biol Chem. 1969;244(2):471–481. - PubMed
-
- Aronson NN, Jr, de Duve C. Digestive activity of lysosomes. II. The digestion of macromolecular carbohydrates by extracts of rat liver lysosomes. J Biol Chem. 1968;243(17):4564–4573. - PubMed
-
- de Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
