A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation
- PMID: 21083419
- PMCID: PMC3117235
- DOI: 10.1089/ten.TEA.2010.0294
A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation
Abstract
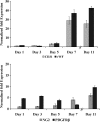
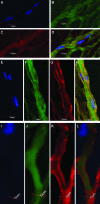
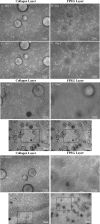
This work describes the differentiation of adipose-derived mesenchymal stem cells (ASC) in a composite hydrogel for use as a vascularized dermal matrix. Our intent is that such a construct could be utilized following large-surface-area burn wounds that require extensive skin grafting and that are limited by the availability of uninjured sites. To develop engineered skin replacement constructs, we are pursuing the use of ASC. We have established that a PEGylated fibrin gel can provide a suitable environment for the proliferation of ASC over a 7 day time course. Further, we have demonstrated that PEGylated fibrin can be used to control ASC differentiation toward vascular cell types, including cells characteristic of both endothelial cells and pericytes. Gene expression analysis revealed strong upregulation of endothelial markers, CD31, and von Willebrand factor, up to day 11 in culture with corresponding evidence of protein expression demonstrated by immunocytochemical staining. ASC were not only shown to express endothelial cell phenotype, but a subset of the ASC expressed pericyte markers. The NG2 gene was upregulated over 11 days with corresponding evidence for the cell surface marker. Platelet-derived growth factor receptor beta gene expression decreased as the multipotent ASC differentiated up to day 7. Increased receptor expression at day 11 was likely due to the enhanced pericyte gene expression profile, including increased NG2 expression. We have also demonstrated that when cells are loaded onto chitosan microspheres and sandwiched between the PEGylated fibrin gel and a type I collagen gel, the cells can migrate and proliferate within the two different gel types. The matrix composition dictates the lineage specification and is not driven by soluble factors. Utilizing an insoluble bilayer matrix to direct ASC differentiation will allow for the development of both vasculature as well as dermal connective tissue from a single population of ASC. This work underscores the importance of the extracellular matrix in controlling stem cell phenotype. It is our goal to develop layered composites as wound dressings or vascularized dermal equivalents that are not limited by nutrient diffusion.
Figures



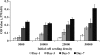



Similar articles
-
Evaluation of gold nanotracers to track adipose-derived stem cells in a PEGylated fibrin gel for dermal tissue engineering applications.Int J Nanomedicine. 2013;8:325-36. doi: 10.2147/IJN.S36711. Epub 2013 Jan 18. Int J Nanomedicine. 2013. PMID: 23345978 Free PMC article.
-
Mechanisms of vasculogenesis in 3D fibrin matrices mediated by the interaction of adipose-derived stem cells and endothelial cells.Angiogenesis. 2014 Oct;17(4):921-33. doi: 10.1007/s10456-014-9439-0. Epub 2014 Aug 3. Angiogenesis. 2014. PMID: 25086616
-
Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force.J Surg Res. 2009 Mar;152(1):157-66. doi: 10.1016/j.jss.2008.06.029. Epub 2008 Jul 26. J Surg Res. 2009. PMID: 19883577 Free PMC article.
-
Understanding the effects of mature adipocytes and endothelial cells on fatty acid metabolism and vascular tone in physiological fatty tissue for vascularized adipose tissue engineering.Cell Tissue Res. 2015 Nov;362(2):269-79. doi: 10.1007/s00441-015-2274-9. Epub 2015 Sep 4. Cell Tissue Res. 2015. PMID: 26340984 Review.
-
Pericytes for the treatment of orthopedic conditions.Pharmacol Ther. 2017 Mar;171:93-103. doi: 10.1016/j.pharmthera.2016.08.003. Epub 2016 Aug 7. Pharmacol Ther. 2017. PMID: 27510330 Free PMC article. Review.
Cited by
-
Biomaterials to prevascularize engineered tissues.J Cardiovasc Transl Res. 2011 Oct;4(5):685-98. doi: 10.1007/s12265-011-9301-3. Epub 2011 Sep 3. J Cardiovasc Transl Res. 2011. PMID: 21892744 Review.
-
Evaluation of gold nanotracers to track adipose-derived stem cells in a PEGylated fibrin gel for dermal tissue engineering applications.Int J Nanomedicine. 2013;8:325-36. doi: 10.2147/IJN.S36711. Epub 2013 Jan 18. Int J Nanomedicine. 2013. PMID: 23345978 Free PMC article.
-
PEGylated Platelet-Free Blood Plasma-Based Hydrogels for Full-Thickness Wound Regeneration.Adv Wound Care (New Rochelle). 2019 Jul 1;8(7):323-340. doi: 10.1089/wound.2018.0844. Epub 2019 Jul 2. Adv Wound Care (New Rochelle). 2019. PMID: 31737420 Free PMC article.
-
Pericyte-like differentiation of human adipose-derived mesenchymal stem cells: An in vitro study.World J Stem Cells. 2020 Oct 26;12(10):1152-1170. doi: 10.4252/wjsc.v12.i10.1152. World J Stem Cells. 2020. PMID: 33178398 Free PMC article.
-
Human adipose-derived cells can serve as a single-cell source for the in vitro cultivation of vascularized bone grafts.J Tissue Eng Regen Med. 2014 Aug;8(8):629-39. doi: 10.1002/term.1564. Epub 2012 Aug 17. J Tissue Eng Regen Med. 2014. PMID: 22903929 Free PMC article.
References
-
- Ehrenreich M. Ruszczak Z. Tissue-engineered temporary wound coverings. Important options for the clinician. Acta Dermatovenerol Alp Panonica Adriat. 2006;15:5. - PubMed
-
- Mansbridge J. Skin tissue engineering. J Biomater Sci Polym Ed. 2008;19:955. - PubMed
-
- Ehrenreich M. Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng. 2006;12:2407. - PubMed
-
- Zhang C.P. Fu X.B. Therapeutic potential of stem cells in skin repair and regeneration. Chin J Traumatol. 2008;11:209. - PubMed
-
- Ogawa R. The importance of adipose-derived stem cells and vascularized tissue regeneration in the field of tissue transplantation. Curr Stem Cell Res Ther. 2006;1:13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

