Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury
- PMID: 21083432
- PMCID: PMC3037803
- DOI: 10.1089/neu.2010.1594
Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury
Abstract
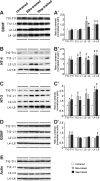
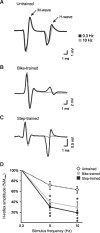
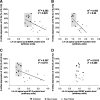
Activity-based therapies such as passive bicycling and step-training on a treadmill contribute to motor recovery after spinal cord injury (SCI), leading to a greater number of steps performed, improved gait kinematics, recovery of phase-dependent modulation of spinal reflexes, and prevention of decrease in muscle mass. Both tasks consist of alternating movements that rhythmically stretch and shorten hindlimb muscles. However, the paralyzed hindlimbs are passively moved by a motorized apparatus during bike-training, whereas locomotor movements during step-training are generated by spinal networks triggered by afferent feedback. Our objective was to compare the task-dependent effect of bike- and step-training after SCI on physiological measures of spinal cord plasticity in relation to changes in levels of neurotrophic factors. Thirty adult female Sprague-Dawley rats underwent complete spinal transection at a low thoracic level (T12). The rats were assigned to one of three groups: bike-training, step-training, or no training. The exercise regimen consisted of 15 min/d, 5 days/week, for 4 weeks, beginning 5 days after SCI. During a terminal experiment, H-reflexes were recorded from interosseus foot muscles following stimulation of the tibial nerve at 0.3, 5, or 10 Hz. The animals were sacrificed and the spinal cords were harvested for Western blot analysis of the expression of neurotrophic factors in the lumbar spinal cord. We provide evidence that bike- and step-training significantly increase the levels of brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and NT-4 in the lumbar enlargement of SCI rats, whereas only step-training increased glial cell-derived neurotrophic factor (GDNF) levels. An increase in neurotrophic factor protein levels that positively correlated with the recovery of H-reflex frequency-dependent depression suggests a role for neurotrophic factors in reflex normalization.
Figures

References
-
- Beaumont E. Houlé J.D. Peterson C.A. Gardiner P.F. Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle Nerve. 2004;29:234–242. - PubMed
-
- Beaumont E. Kaloustian S. Rousseau G. Cormery B. Training improves the electrophysiological properties of lumbar neurons and locomotion after thoracic spinal cord injury in rats. Neurosci. Res. 2008;62:147–154. - PubMed
-
- Bennett D.J. Li Y. Harvey P.J. Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J. Neurophysiol. 2001;86:1972–1982. - PubMed
-
- Boulenguez P. Liabeuf S. Bos R. Bras H. Jean-Xavier C. Brocard C. Stil A. Darbon P. Cattaert D. Delpire E. Marsala M. Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010;16:302–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

