Silymarin use and liver disease progression in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis trial
- PMID: 21083592
- PMCID: PMC3490214
- DOI: 10.1111/j.1365-2036.2010.04503.x
Silymarin use and liver disease progression in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis trial
Abstract
Background: Silymarin is the most commonly used herbal product for chronic liver disease; yet, whether silymarin protects against liver disease progression remains unclear.
Aim: To assess the effects of silymarin use on subsequent liver disease progression in 1049 patients of the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) trial who had advanced fibrosis or cirrhosis and had failed prior peginterferon plus ribavirin treatment.
Methods: Patients recorded their use of silymarin at baseline and were followed up for liver disease progression (two point increase in Ishak fibrosis score across baseline, year 1.5, and year 3.5 biopsies) and over 8.65 years for clinical outcomes.
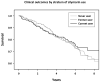
Results: At baseline, 34% of patients had used silymarin, half of whom were current users. Use of silymarin was associated (P < 0.05) with male gender; oesophageal varices; higher ALT and albumin; and lower AST/ALT ratio, among other features. Baseline users had less hepatic collagen content on study biopsies and had less histological progression (HR: 0.57, 95% CI: 0.33-1.00; P-trend for longer duration of use=0.026). No effect was seen for clinical outcomes.
Conclusions: Silymarin use among patients with advanced hepatitis C-related liver disease is associated with reduced progression from fibrosis to cirrhosis, but has no impact on clinical outcomes (Clinicaltrials.gov #NCT00006164).
Published 2010. This article is a US Government work and is in the public domain in the USA.
Conflict of interest statement
Authors with no financial relationships related to this project are: Neal D. Freedman, Teresa M. Curto, Chihiro Morishima, Leonard B. Seeff, Zachary D. Goodman, Elizabeth C. Wright, Rashmi Sinha, James E. Everhart.
Figures

References
-
- Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 (Suppl 1):74–81. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. - PubMed
-
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. - PubMed
-
- Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis. 2007;39:293–304. - PubMed
-
- Verma S, Thuluvath PJ. Complementary and alternative medicine in hepatology: review of the evidence of efficacy. Clin Gastroenterol Hepatol. 2007;5:408–416. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- N01-DK-9-2325/DK/NIDDK NIH HHS/United States
- 1 UL1 RR024986/RR/NCRR NIH HHS/United States
- N01-DK-9-2321/DK/NIDDK NIH HHS/United States
- 1 UL1 RR024982-01/RR/NCRR NIH HHS/United States
- Y99 CA999999/CA/NCI NIH HHS/United States
- N01-DK-9-2320/DK/NIDDK NIH HHS/United States
- M01 RR006192/RR/NCRR NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- M01 RR000633/RR/NCRR NIH HHS/United States
- M01 RR000065/RR/NCRR NIH HHS/United States
- UL1 RR024982/RR/NCRR NIH HHS/United States
- N01-DK-9-2322/DK/NIDDK NIH HHS/United States
- M01 RR000043/RR/NCRR NIH HHS/United States
- 1UL1 RR025758-01/RR/NCRR NIH HHS/United States
- M01 RR000827/RR/NCRR NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- M01RR-00042/RR/NCRR NIH HHS/United States
- 1 UL1 RR 025780-01/RR/NCRR NIH HHS/United States
- M01RR-00065/RR/NCRR NIH HHS/United States
- N01-DK-9-2328/DK/NIDDK NIH HHS/United States
- N01-DK-9-2318/DK/NIDDK NIH HHS/United States
- N01-DK-9-2324/DK/NIDDK NIH HHS/United States
- N01-DK-9-2319/DK/NIDDK NIH HHS/United States
- M01RR-00051/RR/NCRR NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- M01RR-00827/RR/NCRR NIH HHS/United States
- N01-DK-9-2327/DK/NIDDK NIH HHS/United States
- M01RR-01066/RR/NCRR NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- N01-DK-9-2326/DK/NIDDK NIH HHS/United States
- Z99 CA999999/ImNIH/Intramural NIH HHS/United States
- N01-DK-9-2323/DK/NIDDK NIH HHS/United States
- UL1 RR025758/RR/NCRR NIH HHS/United States
- M01 RR000042/RR/NCRR NIH HHS/United States
- M01RR-00633/RR/NCRR NIH HHS/United States
- M01RR-00043/RR/NCRR NIH HHS/United States
- M01RR-06192/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
