Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants
- PMID: 21084117
- PMCID: PMC3033887
- DOI: 10.1016/j.biomaterials.2010.10.052
Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants
Abstract
Drug eluting coatings that can direct the host tissue response to implanted medical devices have the potential to ameliorate both the medical and financial burden of complications from implantation. However, because many drugs useful in this arena are biologic in nature, a paucity of delivery strategies for biologics, including growth factors, currently limits the control that can be exerted on the implantation environment. Layer-by-Layer (LbL) polyelectrolyte multilayer films are highly attractive as ultrathin biologic reservoirs, due to the capability to conformally coat difficult geometries, the use of aqueous processing likely to preserve fragile protein function, and the tunability of incorporation and release profiles. Herein, we describe the first LbL films capable of microgram-scale release of the biologic Bone Morphogenetic Protein 2 (BMP-2), which is capable of directing the host tissue response to create bone from native progenitor cells. Ten micrograms of BMP-2 are released over a period of two weeks in vitro; less than 1% is released in the first 3 h (compared with commercial collagen matrices which can release up to 60% of BMP-2, too quickly to induce differentiation). BMP-2 released from LbL films retains its ability to induce bone differentiation in MC3T3 E1S4 pre-osteoblasts, as measured by induction of alkaline phosphatase and stains for calcium (via Alizarin Red) and calcium matrix (via Von Kossa). In vivo, BMP-2 film coated scaffolds were compared with film coated scaffolds lacking BMP-2. BMP-2 coatings implanted intramuscularly were able to initiate host progenitor cells to differentiate into bone, which matured and expanded from four to 9 weeks as measured by MicroCT and histology. Such LbL films represent new steps towards controlling and tuning host response to implanted medical devices, which may ultimately increase the success of implanted devices, provide alternative new approaches toward bone wound healing, and lay the foundation for development of a multi-therapeutic release coating.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
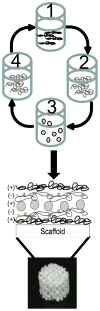



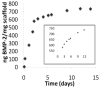



References
-
- Sheridan C. Fresh from the biologic pipeline-2009. Nat Biotechnol. 2009;28(4):307–310. - PubMed
-
- Lavernia CJ, Drakeford MK, Tsao AK, Gittelsohn A, Krackow KA, Hungerford DS. Revision and primary hip and knee arthroplasty - a cost-analysis. Clin Orthop Relat Res. 1995;311:136–141. - PubMed
-
- Fu K, Pack DW, Klibanov AM, Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. 2000;17(1):100–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

