Processing of the synaptic cell adhesion molecule neurexin-3beta by Alzheimer disease alpha- and gamma-secretases
- PMID: 21084300
- PMCID: PMC3024772
- DOI: 10.1074/jbc.M110.142521
Processing of the synaptic cell adhesion molecule neurexin-3beta by Alzheimer disease alpha- and gamma-secretases
Abstract
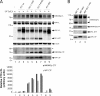
Neurexins (NRXNs) are synaptic cell adhesion molecules having essential roles in the assembly and maturation of synapses into fully functional units. Immunocytochemical and electrophysiological studies have shown that specific binding across the synaptic cleft of the ectodomains of presynaptic NRXNs and postsynaptic neuroligins have the potential to bidirectionally coordinate and trigger synapse formation. Moreover, in vivo studies as well as genome-wide association studies pointed out implication of NRXNs in the pathogenesis of cognitive disorders including autism spectrum disorders and different types of addictions including opioid and alcohol dependences, suggesting an important role in synaptic function. Despite extensive investigations, the mechanisms by which NRXNs modulate the properties of synapses remain largely unknown. We report here that α- and γ-secretases can sequentially process NRXN3β, leading to the formation of two final products, an ∼80-kDa N-terminal extracellular domain of Neurexin-3β (sNRXN3β) and an ∼12-kDa C-terminal intracellular NRXN3β domain (NRXN3β-ICD), both of them being potentially implicated in the regulation of NRXNs and neuroligins functions at the synapses or in yet unidentified signal transduction pathways. We further report that this processing is altered by several PS1 mutations in the catalytic subunit of the γ-secretase that cause early-onset familial Alzheimer disease.
Figures



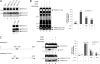

Similar articles
-
Presenilin/γ-secretase regulates neurexin processing at synapses.PLoS One. 2011 Apr 29;6(4):e19430. doi: 10.1371/journal.pone.0019430. PLoS One. 2011. PMID: 21559374 Free PMC article.
-
Shedding of neurexin 3β ectodomain by ADAM10 releases a soluble fragment that affects the development of newborn neurons.Sci Rep. 2016 Dec 19;6:39310. doi: 10.1038/srep39310. Sci Rep. 2016. PMID: 27991559 Free PMC article.
-
Regulated Dynamic Trafficking of Neurexins Inside and Outside of Synaptic Terminals.J Neurosci. 2015 Oct 7;35(40):13629-47. doi: 10.1523/JNEUROSCI.4041-14.2015. J Neurosci. 2015. PMID: 26446217 Free PMC article.
-
Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function.Trends Neurosci. 2006 Jan;29(1):21-9. doi: 10.1016/j.tins.2005.11.003. Epub 2005 Dec 7. Trends Neurosci. 2006. PMID: 16337696 Review.
-
Neuroligins and neurexins link synaptic function to cognitive disease.Nature. 2008 Oct 16;455(7215):903-11. doi: 10.1038/nature07456. Nature. 2008. PMID: 18923512 Free PMC article. Review.
Cited by
-
Neurexin-Neuroligin Synaptic Complex Regulates Schizophrenia-Related DISC1/Kal-7/Rac1 "Signalosome".Neural Plast. 2015;2015:167308. doi: 10.1155/2015/167308. Epub 2015 May 20. Neural Plast. 2015. PMID: 26078884 Free PMC article.
-
Activity-dependent proteolytic cleavage of cell adhesion molecules regulates excitatory synaptic development and function.Neurosci Res. 2017 Mar;116:60-69. doi: 10.1016/j.neures.2016.12.003. Epub 2016 Dec 10. Neurosci Res. 2017. PMID: 27965136 Free PMC article. Review.
-
Proteolytic remodeling of the synaptic cell adhesion molecules (CAMs) by metzincins in synaptic plasticity.Neurochem Res. 2013 Jun;38(6):1113-21. doi: 10.1007/s11064-012-0919-6. Epub 2012 Nov 4. Neurochem Res. 2013. PMID: 23124395 Free PMC article. Review.
-
Differential effects between γ-secretase inhibitors and modulators on cognitive function in amyloid precursor protein-transgenic and nontransgenic mice.J Neurosci. 2012 Feb 8;32(6):2037-50. doi: 10.1523/JNEUROSCI.4264-11.2012. J Neurosci. 2012. PMID: 22323718 Free PMC article.
-
Intercellular signaling by ectodomain shedding at the synapse.Trends Neurosci. 2022 Jun;45(6):483-498. doi: 10.1016/j.tins.2022.03.004. Epub 2022 Apr 13. Trends Neurosci. 2022. PMID: 35430102 Free PMC article. Review.
References
-
- Missler M., Fernandez-Chacon R., Südhof T. C. (1998) J. Neurochem. 71, 1339–1347 - PubMed
-
- Rowen L., Young J., Birditt B., Kaur A., Madan A., Philipps D. L., Qin S., Minx P., Wilson R. K., Hood L., Graveley B. R. (2002) Genomics 79, 587–597 - PubMed
-
- Missler M., Südhof T. C. (1998) Trends Genet 14, 20–26 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

