Up-regulation of ectopic trypsins in the myocardium by influenza A virus infection triggers acute myocarditis
- PMID: 21084314
- PMCID: PMC3028976
- DOI: 10.1093/cvr/cvq358
Up-regulation of ectopic trypsins in the myocardium by influenza A virus infection triggers acute myocarditis
Abstract
Aims: Influenza A virus (IAV) infection markedly up-regulates ectopic trypsins in various organs, viral envelope glycoprotein processing proteases, which are pre-requisites for virus entry and multiplication. We investigated the pathological roles of trypsin up-regulation in the progression of IAV-induced myocarditis, cytokine induction, and viral replication in the hearts, and also investigated the protective effects of trypsin inhibitor on cardiac dysfunction in vivo and selective knockdown of trypsin on IAV-induced cellular damage in cardiomyoblasts.
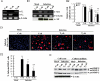
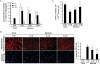
Methods and results: The relationship of the expression among IAV RNA, trypsins, matrix metalloproteinase (MMP)-9, MMP-2, pro-inflammatory cytokines interleukin (IL)-6, IL-1β, and tumour necrosis factor-α was analysed in mice hearts and cardiomyoblasts after IAV infection. The severity of myocarditis was most noticeable during Day 6-9 post-infection, along with peak expression of viral RNA, trypsins, particularly trypsin₂, MMPs, and cytokines. Cardiac ATP levels were the lowest at Day 9. Up-regulated trypsins, viral protein, and tissue-injured loci in the myocardium were closely localized. Trypsin inhibitor aprotinin treatment in vivo and selective trypsin₁- and trypsin₂-knockdown, particularly the latter, in H9c2 cardiomyoblasts significantly suppressed viral replication, up-regulation of MMPs, and production of active MMP-9 and cytokines, resulting in marked protection against cellular damage, ATP depletion, and apoptosis. IAV infection-induced cardiac dysfunction monitored by echocardiography was improved significantly by aprotinin treatment.
Conclusions: IAV-induced trypsins, particularly trypsin₂, in the myocardium trigger acute viral myocarditis through stimulation of IAV replication, proMMP-9 activation, and cytokine induction. These results suggest that up-regulation of trypsins is one of the key host pathological findings in IAV-induced myocarditis.
Figures




Comment in
-
What we learned from pandemic H1N1 influenza A.Cardiovasc Res. 2011 Feb 15;89(3):483-4. doi: 10.1093/cvr/cvq407. Epub 2010 Dec 22. Cardiovasc Res. 2011. PMID: 21177702 No abstract available.
References
-
- Kim HW, Brandt CD, Arrobio JO, Murphy B, Chanock RM, Parrott RH. Influenza A and B virus infection in infants and young children during the years 1957–1976. Am J Epidemiol. 1979;109:464–479. - PubMed
-
- Mamas MA, Fraser D, Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol. 2008;130:304–309. - PubMed
-
- Warren-Gash C, Smeeth L, Hayward AC. Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: a systemic review. Lancet Infect Dis. 2009;10:601–610. - PubMed
-
- Davis MM, Taubert K, Benin AL, Brown DW, Mensah GA, Baddour LM, et al. Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. Circulation. 2006;114:1549–1553. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

