Nonlinear speech analysis algorithms mapped to a standard metric achieve clinically useful quantification of average Parkinson's disease symptom severity
- PMID: 21084338
- PMCID: PMC3104343
- DOI: 10.1098/rsif.2010.0456
Nonlinear speech analysis algorithms mapped to a standard metric achieve clinically useful quantification of average Parkinson's disease symptom severity
Abstract
The standard reference clinical score quantifying average Parkinson's disease (PD) symptom severity is the Unified Parkinson's Disease Rating Scale (UPDRS). At present, UPDRS is determined by the subjective clinical evaluation of the patient's ability to adequately cope with a range of tasks. In this study, we extend recent findings that UPDRS can be objectively assessed to clinically useful accuracy using simple, self-administered speech tests, without requiring the patient's physical presence in the clinic. We apply a wide range of known speech signal processing algorithms to a large database (approx. 6000 recordings from 42 PD patients, recruited to a six-month, multi-centre trial) and propose a number of novel, nonlinear signal processing algorithms which reveal pathological characteristics in PD more accurately than existing approaches. Robust feature selection algorithms select the optimal subset of these algorithms, which is fed into non-parametric regression and classification algorithms, mapping the signal processing algorithm outputs to UPDRS. We demonstrate rapid, accurate replication of the UPDRS assessment with clinically useful accuracy (about 2 UPDRS points difference from the clinicians' estimates, p<0.001). This study supports the viability of frequent, remote, cost-effective, objective, accurate UPDRS telemonitoring based on self-administered speech tests. This technology could facilitate large-scale clinical trials into novel PD treatments.
© 2010 The Royal Society
Figures

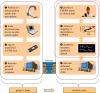
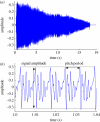

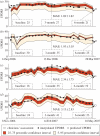
Similar articles
-
Accurate telemonitoring of Parkinson's disease progression by noninvasive speech tests.IEEE Trans Biomed Eng. 2010 Apr;57(4):884-93. doi: 10.1109/TBME.2009.2036000. Epub 2009 Nov 20. IEEE Trans Biomed Eng. 2010. PMID: 19932995
-
Estimation of Parkinson's disease severity using speech features and extreme gradient boosting.Med Biol Eng Comput. 2020 Nov;58(11):2757-2773. doi: 10.1007/s11517-020-02250-5. Epub 2020 Sep 10. Med Biol Eng Comput. 2020. PMID: 32910301
-
Remote Assessment of Parkinson's Disease Symptom Severity Using the Simulated Cellular Mobile Telephone Network.IEEE Access. 2021 Jan 11;9:11024-11036. doi: 10.1109/ACCESS.2021.3050524. eCollection 2021. IEEE Access. 2021. PMID: 33495722 Free PMC article.
-
H. pylori and Parkinson's disease: Meta-analyses including clinical severity.Clin Neurol Neurosurg. 2018 Dec;175:16-24. doi: 10.1016/j.clineuro.2018.09.039. Epub 2018 Oct 1. Clin Neurol Neurosurg. 2018. PMID: 30308319 Review.
-
Technologies for Assessment of Motor Disorders in Parkinson's Disease: A Review.Sensors (Basel). 2015 Aug 31;15(9):21710-45. doi: 10.3390/s150921710. Sensors (Basel). 2015. PMID: 26404288 Free PMC article. Review.
Cited by
-
Quantifying short-term dynamics of Parkinson's disease using self-reported symptom data from an Internet social network.J Med Internet Res. 2013 Jan 24;15(1):e20. doi: 10.2196/jmir.2112. J Med Internet Res. 2013. PMID: 23343503 Free PMC article.
-
A machine learning method to process voice samples for identification of Parkinson's disease.Sci Rep. 2023 Nov 23;13(1):20615. doi: 10.1038/s41598-023-47568-w. Sci Rep. 2023. PMID: 37996478 Free PMC article.
-
Detecting Parkinson Disease Using a Web-Based Speech Task: Observational Study.J Med Internet Res. 2021 Oct 19;23(10):e26305. doi: 10.2196/26305. J Med Internet Res. 2021. PMID: 34665148 Free PMC article.
-
Will Artificial Intelligence Replace the Movement Disorders Specialist for Diagnosing and Managing Parkinson's Disease?J Parkinsons Dis. 2021;11(s1):S117-S122. doi: 10.3233/JPD-212545. J Parkinsons Dis. 2021. PMID: 34219671 Free PMC article. Review.
-
Towards the identification of Idiopathic Parkinson's Disease from the speech. New articulatory kinetic biomarkers.PLoS One. 2017 Dec 14;12(12):e0189583. doi: 10.1371/journal.pone.0189583. eCollection 2017. PLoS One. 2017. PMID: 29240814 Free PMC article.
References
-
- von Campenhausen S., et al. 2005. Prevalence and incidence of Parkinson's disease in Europe. Eur. Neuropsychopharmacol. 15, 473–49010.1016/j.euroneuro.2005.04.007 (doi:10.1016/j.euroneuro.2005.04.007) - DOI - DOI - PubMed
-
- Schrag A., Ben-Schlomo Y., Quinn N. 2002. How valid is the clinical diagnosis of Parkinson's disease in the community? J. Neurol. Neurosurg. Psychiatry 73, 529–53510.1136/jnnp.73.5.529 (doi:10.1136/jnnp.73.5.529) - DOI - DOI - PMC - PubMed
-
- Elbaz A., Bower J. H., Maraganore D. M., McDonnell S. K., Peterson B. J., Ahlskog J. E., Schaid D. J., Rocca W. A. 2002. Risk tables for Parkinsonism and Parkinson's disease. J. Clin. Epidemiol. 55, 25–3110.1016/S0895-4356(01)00425-5 (doi:10.1016/S0895-4356(01)00425-5) - DOI - DOI - PubMed
-
- Pahwa R., Lyons E. 2007. Handbook of Parkinson's disease, 4th edn New York, NY: Informa Healthcare
-
- Harel B., Cannizzaro M., Snyder P. J. 2004. Variability in fundamental frequency during speech in prodromal and incipient Parkinson's disease: a longitudinal case study. Brain Cogn. 56, 24–2910.1016/j.bandc.2004.05.002 (doi:10.1016/j.bandc.2004.05.002) - DOI - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

