The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle
- PMID: 21084444
- PMCID: PMC3033058
- DOI: 10.1210/en.2010-0802
The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle
Abstract
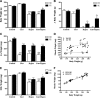
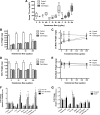
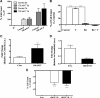
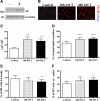
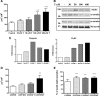
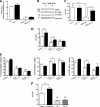
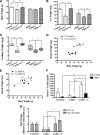
Testosterone (T) supplementation increases skeletal muscle mass, circulating GH, IGF-I, and im IGF-I expression, but the role of GH and IGF-I in mediating T's effects on the skeletal muscle remains poorly understood. Here, we show that T administration increased body weight and the mass of the androgen-dependent levator ani muscle in hypophysectomized as well as castrated plus hypophysectomized adult male rats. T stimulated the proliferation of primary human skeletal muscle cells (hSKMCs) in vitro, an effect blocked by transfecting hSKMCs with small interference RNA targeting human IGF-I receptor (IGF-IR). In differentiation conditions, T promoted the fusion of hSKMCs into larger myotubes, an effect attenuated by small interference RNA targeting human IGF-IR. Notably, MKR mice, which express a dominant negative form of the IGF-IR in skeletal muscle fibers, treated with a GnRH antagonist (acyline) to suppress endogenous T, responded to T administration by an attenuated increase in the levator ani muscle mass. In conclusion, circulating GH and IGF-I are not essential for mediating T's effects on an androgen-responsive skeletal muscle. IGF-I signaling plays an important role in mediating T's effects on skeletal muscle progenitor cell growth and differentiation in vitro. However, IGF-IR signaling in skeletal muscle fibers does not appear to be obligatory for mediating the anabolic effects of T on the mass of androgen-responsive skeletal muscles in mice.
Figures
References
-
- Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ 2002 Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282:601–607 - PubMed
-
- Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S 2002 Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab 283:154–164 - PubMed
-
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW 2005 Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 90:678–688 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

