Targeted inactivation of the androgen receptor gene in murine proximal epididymis causes epithelial hypotrophy and obstructive azoospermia
- PMID: 21084446
- PMCID: PMC3101806
- DOI: 10.1210/en.2010-0768
Targeted inactivation of the androgen receptor gene in murine proximal epididymis causes epithelial hypotrophy and obstructive azoospermia
Abstract
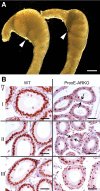
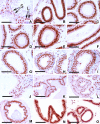
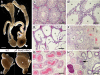
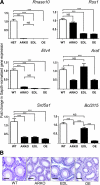
The epithelial lining of the epididymal duct expresses the androgen receptor (Ar) along its entire length and undergoes rapid and profound degeneration when androgenic support is withdrawn. However, experiments involving orchidectomy with systemic testosterone replacement, and testicular efferent duct ligation, have indicated that structural and functional integrity of the initial segment cannot be maintained by circulating androgen alone, leaving the role of androgen in this epididymal zone unclear. We addressed this question in a mouse model with intact testicular output and selective Ar inactivation in the proximal epididymis by creating double-transgenic males carrying a conditional Ar(loxP) allele and expressing Cre recombinase under the promoter of Rnase10, a gene specifically expressed in proximal epididymis. At 20-25 d of life, on the onset of Rnase10 expression, Ar became selectively inactivated in the principal cells of proximal epididymis, resulting in epithelial hypoplasia and hypotrophy. Upon the subsequent onset of spermiation, epididymal obstruction ensued, with the consequent development of spermatic granulomata, back pressure-induced atrophy of the seminiferous epithelium, orchitis, and fibrosis of the testicular parenchyma. Consistent with these findings, the mice were infertile. When the effect of Ar knockout on gene expression in the proximal epididymis was compared with that of efferent duct ligation and orchidectomy, we identified genes specifically regulated by androgen, testicular efferent fluid, and both. Our findings demonstrate that the development and function of the epididymal initial segment is critically dependent on direct androgen regulation. The phenotype of the produced knockout mouse provides a novel model for obstructive azoospermia.
Figures

References
-
- Robaire B, Hinton BT, Orgebin-Crist MC. 2006. The epididymis. In: Neill JD. ed. Knobil and Neilly's physiology of reproduction. Vol 1 San Diego: Elsevier Academic Press; 1071–1148
-
- Fawcett DW, Hoffer AP. 1979. Failure of exogenous androgen to prevent regression of the initial segments of the rat epididymis after efferent duct ligation or orchidectomy. Biol Reprod 20:162–181 - PubMed
-
- Hamzeh M, Robaire B. 2009. Effect of testosterone on epithelial cell proliferation in the regressed rat epididymis. J Androl 30:200–212 - PubMed
-
- Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA. 2002. Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl 23:870–881 - PubMed
-
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lécureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. 2004. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101:1327–1332 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

